Published by: BhumiRaj Timalsina
Published date: 23 Jun 2021
The Zeeman Effect is one of the experiments included in Space Quantization which is described below.
Picture
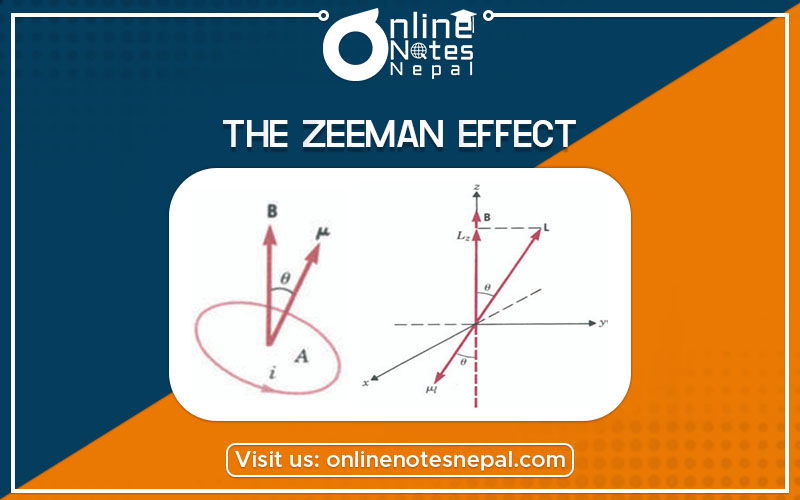
We know that if a current i flows through a loop of area A, a magnetic dipole moment μ = iA is associated with it If we place this dipole in an external uniform magnetic field B it will experience a torque τ = μ x B = μ B sin θ, where θ is the angle between the two vectors.
The torque is a maximum when the dipole is perpendicular to the field and zero when it is parallel or antiparallel to it. As a consequence, we can associate with such a dipole μ in a magnetic field B potential energy that will depend on the orientation of μ with respect to B. The potential energy Ep is
equation
Picture
Let us consider an electron in one of the Bohr orbits as shown in the above figure; the circulating electron represents a current i given by
equation
where T is the period of rotation, v is the tangential speed of the electron, and r is the radius of the orbit. Then
equation
In quantum mechanics we cannot talk about a particle moving in a circular loop of radius r with a velocity v. We can rewrite this expression in a form that is meaningful quantum mechanically by multiplying the numerator and the denominator by the mass of the electron and recalling that mvr is the angular momentum L of a particle of mass m rotating with a tangential velocity v about a point a distance r away.
equation
We have included a minus sign because the direction of i is opposite to the direction of motion of the electron, hence of L and we have written μ with the subscript I to indicate that the dipole is associated with the orbital motion of the electron. Thus, we have established that an atom with angular momentum L has associated with it a magnetic dipole moment. If we now place the atom in a magnetic field B, there will be an extra contribution to the energy
equation
Picture
From
L cos θ= Lz therefore
equation
The total energy of the atom, Etotal will be the sum of the energy resulting from the interaction of the electron with the nucleus, that is, and the energy resulting from the interaction of the dipole moment with the magnetic field B,
equation
Classically, Lz can take any value between Land – L. This means that an energy level En would become a band of width 2 (|e|/2m) (BL). Quantum mechanically, however, Lz can take only certain values, that is, Lz = mlћ Thus the total energy will be given by
equation
Because ml takes values 0, ± I, ± 2, . . . ± I, the energy levels of quantum states with the orbital quantum number I larger than 0 and thus, with several values for mil split into several discrete sublevels If we now reexamine the spectrum of hydrogen, the n = 2 to n = 1 transition should give rise to three different lines (three different A’s). In the transitions from higher n states, we may expect even a greater number of lines. However, there is a selection rule that initially was found experimentally and was later derived from the Schrodinger theory which states that the only allowed transitions are those for which ml = 0 or ± 1.
equation
Thus, regardless of the number of sublevels, transitions from a given n to another should give rise to only three distinct lines. This effect is known as The Normal Zeeman Effect.
It had been observed experimentally in the spectrum of such elements as helium, calcium, zinc, and a few others long before quantum mechanics was formulated.
Often more than three lines are observed. For example, in hydrogen the n = 2 to n = 1 transition shows seven closely spaced lines. This effect is known as The Anomalous Zeeman Effect. Thus, the experiment shows that the idea of space quantization is correct. However, it also shows that the Schrodinger theory as originally formulated was incomplete.
If you liked our content The Zeeman Effect then please don’t forget to check our other topics Schrodinger Equation for The H Atom