Published by: BhumiRaj Timalsina
Published date: 26 Jun 2021
Photoconductivity is the tendency to conduct electricity to a point that depends on the intensity of light radiant striking the surface of an object. Most of the semiconductors materials do have this property. So, we use this technique to measure the energy gap of the semiconductor. When we expose the semiconductor to the different frequencies of photons, we observe the ability of the object to absorb them.
When a semiconductor material absorbs a beam of light, the number of free electrons and electron holes increases and raises the electrical conductivity. For causing the excitation, the light that strikes the surface of the semiconductor must have enough energy to raise the electrons across the bandgap.
When there is a connection of photoconductive material as a part of the circuit, it functions as a resistor whose resistance depends on the light intensity. In this condition, the material is a photoresistor. The use of these devices is as photodetectors which measure the light intensity.
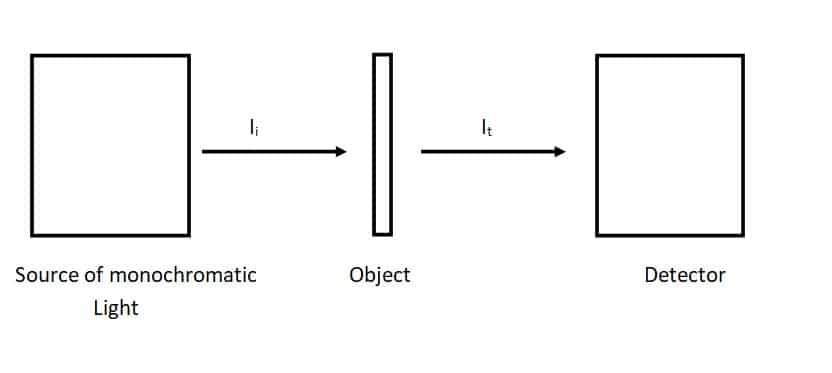
Let the light from a monochromator strikes a thin slab of the material. We measure the transmitted radiation by a light meter. The amount of absorbed radiation can be found by subtracting the intensity of the transmitted beam from the incident beam. The semiconductors show negligible absorption for long wavelengths (i.e. small frequency) and then give it a sudden rise. This is the absorption edge.
If the energy of the photons ’hf’ is less than that of the energy of the gap Eg, the electrons will not be able to excite from the valence band into the conduction band; the electrons will not absorb photons but will go through the object. Hence, the minimum frequency fc that causes photoconduction is,
hfc = Eg
For Si, λc = 1.13 x 10-6 m
Eg = {(6.63 x 10-34 J-sec) (3 x 108 m/sec)}/1.13 x 10-6 m
= 1.8 x 10-19 J
= 1.1 eV
Some of the examples of photodetectors are charge-coupled devices (CCDs), photodiodes, phototransistors, etc.