Published by: BhumiRaj Timalsina
Published date: 25 Jun 2021
Assumptions of Classical Free Electron Model are as follows;
Where, k = Boltzmann Constant and T = Absolute Temperature.
It is used to derive Ohm’s law as follows;
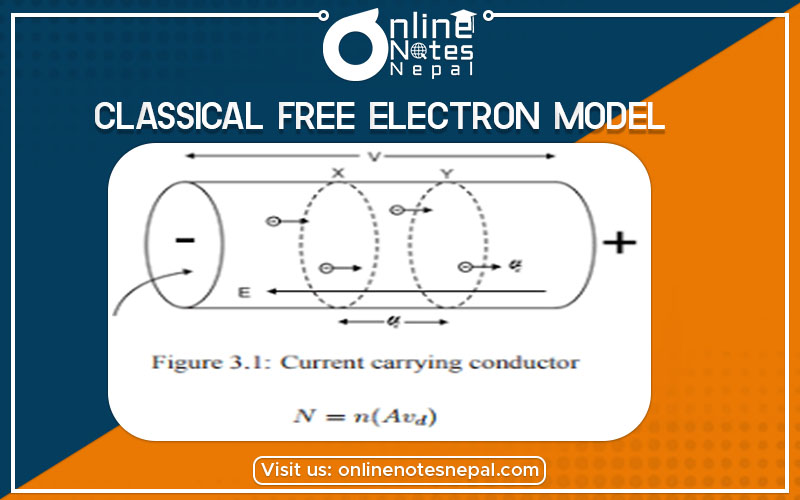
Let us consider a cylindrical metallic conductor having length ‘l’ and cross-sectional area ‘A’. Again, n is the number of free electrons per unit volume. If ‘v’ is the velocity of an electron moving with distance ‘x’ in time ‘t’, then,
Distance(x) = vd × t
Total number of electron(N) = n × vol.
Or, N = n × A × distance
= n × A × vd × t ……(i)
Now, current is rate of flow of charge,
I = Q/t
i.e. I = Ne/t ……(ii)
From equation (i) and (ii)
I = (n × A × vd × t × e)/t
i.e. I = vdenA …..(iii)
Now, Current Density (J) = I/A = (vdenA)/A
Therefore, J = vden ……(iv)
If Fe is the electric force then,
Fe= Ee
Or, ma = eE (F=ma)
Or, a = eE/m
Or, dv/dt = eE/m
Or, dv = dt. eE/m
Integrating on both sides we get,
Vd = eEτ/m …….(v)
Where τ = Relaxation Time
From (iv) and (v)
J = vden
= (eEτ × en)/m
= (ne2τ × E)/m
Hence,
[i.e. (ne2τ)/m = σ = electrical conductivity]
Which is Ohm’s law.
1) Specific head:-
The molar specific heat of a gas at constant volume is cv= 2/2 RT but experimentally it was found that the specific heat of a metal by its conduction e was CV=10-4 RT which shows dependence on temperature which is contra vary to the theory.
2)The temperature-dependent electrical conductivity of a metal experimentally was found that
Expt𝜎 ∝⊥/𝑇
The theoretical was found that
Theort𝜎 ∝⊥/√𝑇
3)Dependence of electrical conductivity on electron concentration.
4) 𝜎 =ne2T/m
n—> electron concentration.
Cu 8.2×1028 e/m3
A1 18.06X1028 e/m3
A1 > CU—> classical theory
Cu̇ 𝜎 A1 experimentally