Published by: BhumiRaj Timalsina
Published date: 26 Jun 2021
The actual crystal bonding is usually a mixture of two or three. There are four general types of bonding ionic bond, covalent bond, metallic bond, and molecular bond. As mentioned before, the exact knowledge of the crystal structure is not necessary in order to obtain a semi-quantitative understanding of conduction processes in solids. However, the scientist who wants to make exact calculations of some property of a particular crystal must have a detailed knowledge of the crystal structure of that material. For our purposes it is more important to achieve an understanding of the different mechanisms that hold the structure together, that is, the different types of bonding. that we discuss next.
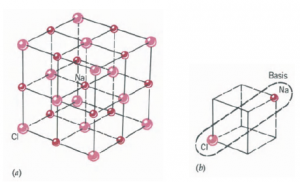
Ionic crystals consist of positive and negative ions in a periodic array. This is a result of an atom losing one or more electrons and another atom capturing them. For this to occur, the exchange must be energetically favorable. This means that if one starts with two neutral atoms, say Na and Cl, and goes through the process of ionizing one of them, then giving the resulting electron or electrons to the other and bringing the two ions together,
the overall energy of the bound pair must be less than the energy of the initial state of two neutral atoms. To ionize Na, the energy of 5.14 eV must be provided, which may be written as
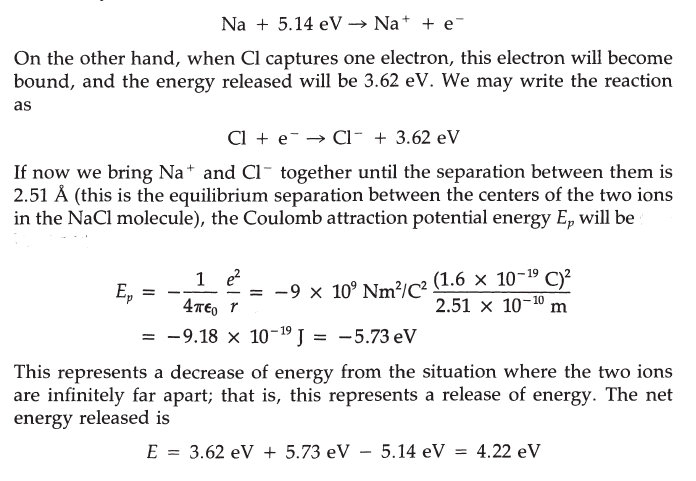
If E = 0 is the reference energy state when the Na and Cl atoms are far apart, when they are ions at r = 2.51 Their energy is -4.22 eV. In other words, they fall into a potential well of depth -4.22 eV. This is the energy needed to break apart the NaCl molecule and restore each ion to electrical neutrality. In an ionic crystal, the binding energy is the sum of all the attractive forces (between all ions of opposite sign) and all the repulsive forces (between ions of like sign). For an ionic crystal, this energy can be written as

Another important and very strong type of bond is the covalent bond. A good example of this is the H2 molecule. The bond comes about because both electrons are in orbit (so to speak) around both nuclei. Because electrons repel each other, the largest average distance apart can be maintained when their motions are coupled so that at a given time each is on the same side of each nucleus.
Therefore, one or the other of the electrons spends part of its time between the two nuclei, attracting both of them and thus binding them. One way to understand why this takes place is by considering the tendency of the atoms to have filled subshells. The hydrogen atom needs one more electron to fill the Is subshell and thus achieve the inert electronic configuration of helium. Lacking any other source of electrons, it will try to capture the extra electron from the adjacent hydrogen atom. Of course, the other atom will try to do the same, and they compromise by sharing both electrons.
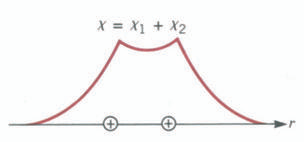
This can be put into a more rigorous quantum mechanical language. Consider two identical H atoms, each with its electron in the Is state. When the two atoms are far apart the wave functions do not overlap, and therefore the electrons remain with their parent atoms. The situation is shown in Figure below, which represents the radial dependence of the Is wave function for hydrogen atoms numbered 1 and 2. Note that each wave function is radially symmetric about a nucleus.
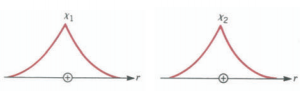
If, however, the two atoms are brought close together until the nuclear separation is = 0.7 A, the two wave functions overlap significantly. Because the electron from one atom can be found close to the nucleus of the other, it can be captured by the latter, and as a result, for a certain fraction of its time a given electron will be found with each proton. Because the two electrons are indistinguishable, it can be said that they spend equal time with each proton. The one that leads to the covalent bond is the sum of the two wave functions X = Xl + X2.
The best examples of covalent bonding in solids are offered by carbon in the diamond structure, silicon, and germanium. These elements belong to group IV of the periodic table. The outermost electron configuration consists of a filled s subshell followed by a p subshell with two electrons (C: 1s2 2s2 2p2; Si: 1s2 2s2 2p6 3s2 3p2; Ge: [Ar] 3d¹⁰ 4s² 4p²) . To have a full p subshell, each needs four more electrons. They achieve this by sharing four electrons with four other atoms. Each atom provides two electrons from the outer p subshell and two from the s subshell in order to form four covalent bonds. Even though the two electrons in the s subshell form a closed subshell, they participate in the bonding because they are very close in energy to the next p subshell. The most stable arrangement of the four bonds occurs when the atoms are symmetrically arranged in space. This symmetry is achieved by having a tetrahedral structure. Each atom is at the center of a tetrahedron and makes a covalent bond with its four nearest neighbors, which are located at the corners of the tetrahedron. Each corner atom, in turn, finds itself at the center of another tetrahedron, so that the structure consists of a system of interlocked tetrahedra. The structure is in fact two interpenetrating face-centered cubic lattices that are displaced along the diagonal by one a quarter of the diagonal of the cube.