Published by: BhumiRaj Timalsina
Published date: 30 Jun 2021
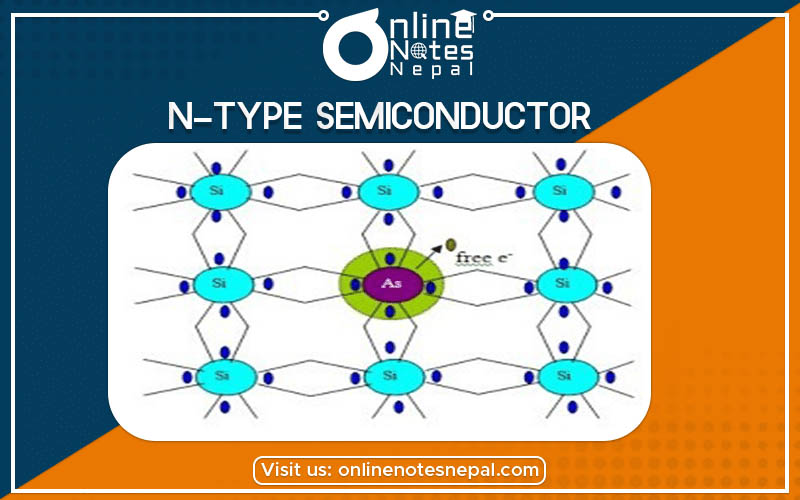
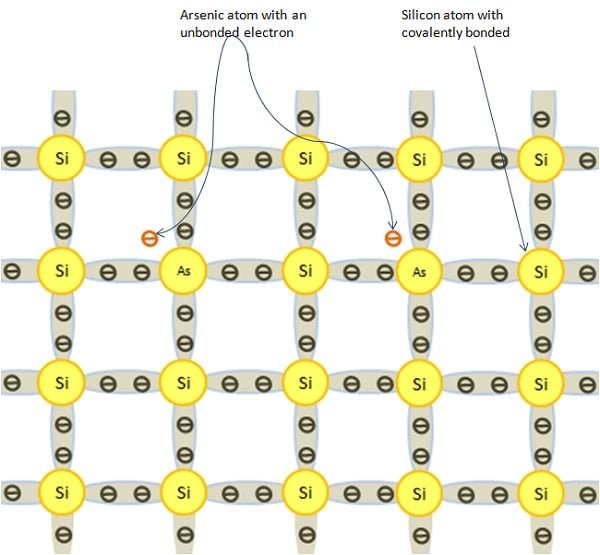
N-type semiconductors are those semiconductors that are made when a pentavalent impurity is added to the pure semiconductor to increase the conductivity. The pentavalent elements are Silicon (Si), Arsenic (As), Phosphorous (P), Antimony (Sb), etc. The four out of the five valence electron of these impurities make the covalent bond with four electrons of silicon, so one electron remains free. This happens in every addition of impurity. These are donated by the impurity atom. For this reason, Energy level E0 is known as the donor level. When there is the ionization of the donor level, no hole is created in the valence band. This extra revolves around the impurity ion core like the electron in the hydrogen atom.
The binding energy of the electron in the hydrogen atom is,
Eb = (me e4)/ (8 C02h2)
= 13.6 eV
To free the extra electron from the impurity iron core, the energy required is,
EbSi = (me*e4)/ (8ε2h2)
= (me*e4)/ (8ε02 εr2h2)
= 0.032 eV
Where,
Effective mass of electron me* = (me)/ 3 and εr = 11.9
Now, this energy is comparable to the average thermal energy of the atomic variation at room temperature ~ 3/2 KT (~0.035eV). The fifth valence electron can be readily freed by the thermal vibration of the Si lattice.
In the n-type, the concentration of electron increases with every addition of the impurity. So, it is a negative n-type semiconductor. The pentavalent atom donates the electron for conduction, hence it is the donor. In the n-type, electrons are the major charge carriers and holes are the minor charge carriers.
If n and p represent extrinsic electron and hole concentration of semiconductor, Nd the donor concentration, then let us suppose all the donor sites are ionized,
n = p + Nd
But,
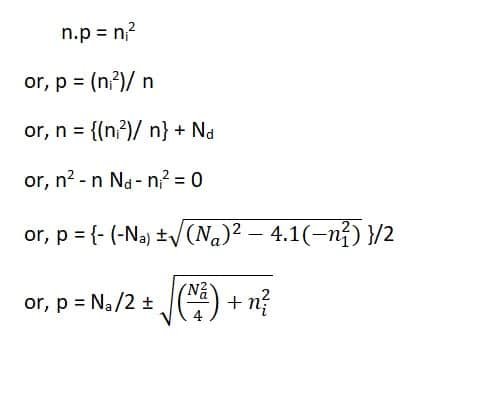
Now, ignoring the –ve sign and for (Nd2)/ 4 >> ni2
n ~ (Nd /2) + (Nd /2)
~ Nd
Therefore, it proves that in an n-type, the electron concentration is nearly equal to the donor concentration.
Here,
n.p = ni2
p = (ni2)/ n
= (ni2)/ Nd
Therefore, hole concentration in the n-type semiconductor is p ~ (ni2)/ Nd
The hole concentration in the n-type semiconductor is less than that of the intrinsic hole concentration. This happens because some electrons in the conduction band recombine with the holes to maintain n.p = ni2. As the concentration of donor atoms goes on increasing it increases the majority carrier concentration. It decreases the minority carrier concentration simultaneously.
The conductivity of the semiconductor is,
σ = n e µe + p e µh
= Nd e µe + {(ni2)/ Nd} e µh
σ = Nd e µe
It shows that conductivity is due to mobilities of the electron in the n-type.