Published by: BhumiRaj Timalsina
Published date: 30 Jun 2021
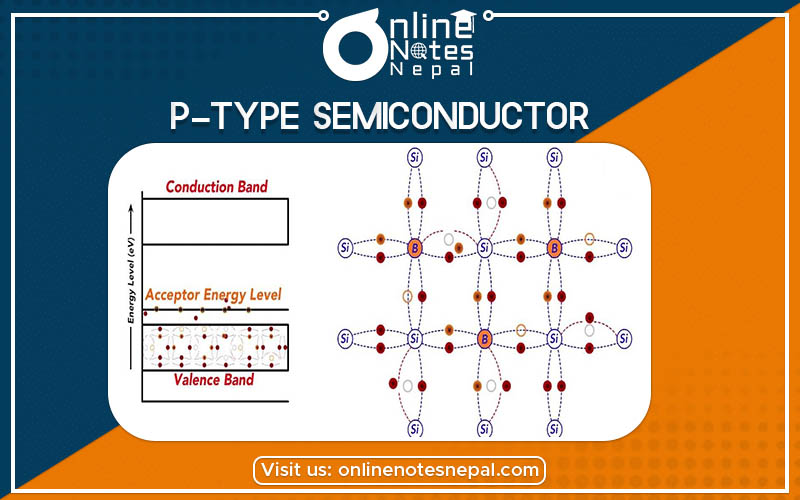
P-type semiconductors are those extrinsic semiconductors doped with a trivalent impurity element which consists of three electrons in its valence shell. These pentavalent impurities increase the number of electrons for the conduction.
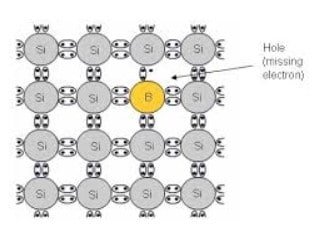
The three valence electron of these impurities form only three complete covalent bonds with silicon which has four valence electrons. There becomes a deficiency of one electron to form the fourth bond. This creates a hole.
A nearby electron tunnels into the hole and displaces the hole further away from the Boron atom. As the hole moves away, it gets attracted by the negative charge left behind on the Boron. It then takes into orbit around the B– ions. The binding energy of the hole to the B– ion can be calculated using the hydrogenic atom analogy. In these types of semiconductors, the concentration of the hole increases with every addition of impurity. These are p-type semiconductors.
Let n and p be the electrons and hole concentration in the semiconductor and Na is the acceptor concentration. The doping of trivalent impurities increases the hole concentration of the valence band but does not increase the electron concentration in the conduction band. So,
p = Na + n
Since,
n.p = ni2
n = ni2 / p
Therefore,
p = Na + ni2 / p
or, p = (p Na + ni2) / p
or, p2 – p Na – ni2 = 0
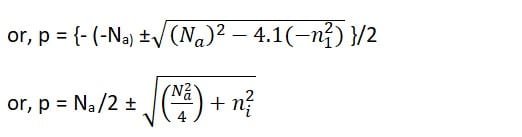
Ignoring the negative sign and for (Na2/4) >> ni2
p = (Na /2) + (Na /2)
= Na
Therefore in the p-type semiconductor hole concentration is nearly equal to the acceptor concentration.
Here,
n.p = ni 2
n = ni 2 / p = ni 2 / Na
Therefore, electron concentration in the p-type semiconductor is,
n = ni 2 / Na
In the trivalent doping of semiconductor, the hole concentration increases and electron concentration decreases.