Published by: BhumiRaj Timalsina
Published date: 23 Jun 2021
All objects with a temperature above absolute zero (0 K, -273.15 oC) emit energy in the form of electromagnetic radiation.
A blackbody radiation is a theoretical or model body that absorbs all radiation falling on it, reflecting or transmitting none. It is a hypothetical object which is a “perfect” absorber and a “perfect” emitter of radiation over all wavelengths.
A perfect black body is an ideal conception. There is no known surface which can be regarded as a perfectly black body. Lampblack or platinum black is the nearest approach to a perfectly black body. Lampblack can absorb about 96% of the radiation incident on it, and platinum black absorbs about 98%.
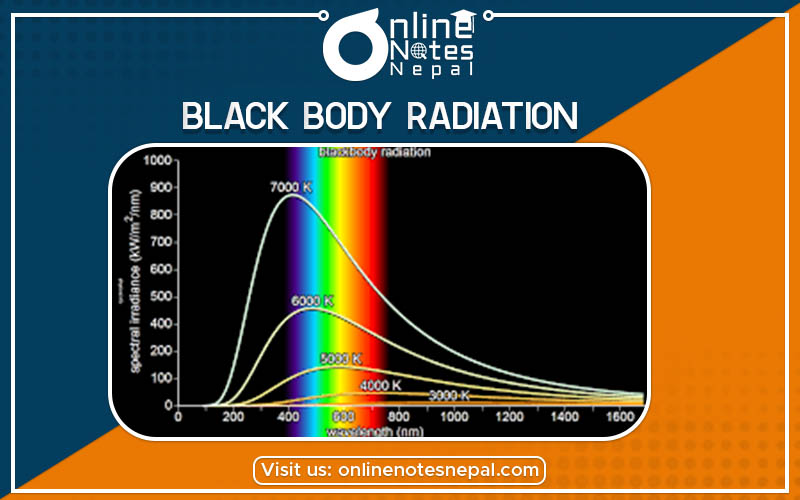
The spectral distribution of the thermal energy radiated by a blackbody (i.e. the pattern of the intensity of the radiation over a range of wavelengths or frequencies) depends only on its temperature.
Photo
Fig: Black body radiation curves at different temperature curves
The main features of the spectrum emitted bt a black body are;
In order to explain the distribution of energy in the spectrum of a blackbody, Max Planck in 1990, put forward the quantum theory of radiation. He assumed that the atoms in the walls of a blackbody behave like simple harmonic oscillators, and each has a characteristic frequency of oscillation. In his theory, he made two radical assumptions about the atomic oscillator:
Photo
Meanwhile, the energy of radiation is expressed in terms of frequency as,
E = h ν
Where,
E = Energy of the radiation
h = Planck’s constant (6.626×10–34 J.s)
ν= Frequency of radiation
Interestingly, Planck has also concluded that these were only an aspect of the processes of absorption and emission of radiation. They had nothing to do with the physical reality of the radiation itself. Later in the year 1905, famous German physicist, Albert Einstein also reinterpreted Planck’s theory to further explain the photoelectric effect. He was of the opinion that if some source of light was focused on certain materials, they can eject electrons from the material. Basically, Planck’s work led Einstein in determining that light exists in discrete quanta of energy, or photons.
If you liked our content Boolean Algebra, then please don’t forget to check our other topics Rotational Kinetic Energy.