Published by: Zaya
Published date: 22 Jun 2021
CLASSICAL FREE ELECTRON (CFE) MODEL
Many of the difficulties encountered by the classical free electron model were removed with the Quantum-Mechanical Free Electron Model. the classical free electron model. The first successful attempt to understand the electrical properties of metals was presented by P. Drude in 1900 and was extended by H. A. Lorentz in 1909. The results of these two investigators, as well as the work of others, is now called the classical free electron model (CFE).
Assumptions of the Model
½ mv-2= (3/2)kT
where v-2 is the average of the square of the thermal speeds; k is Boltzmann's constant, 1.38 x 10- 23 J/K; and T is the absolute temperature of the solid and therefore of the electron gas.
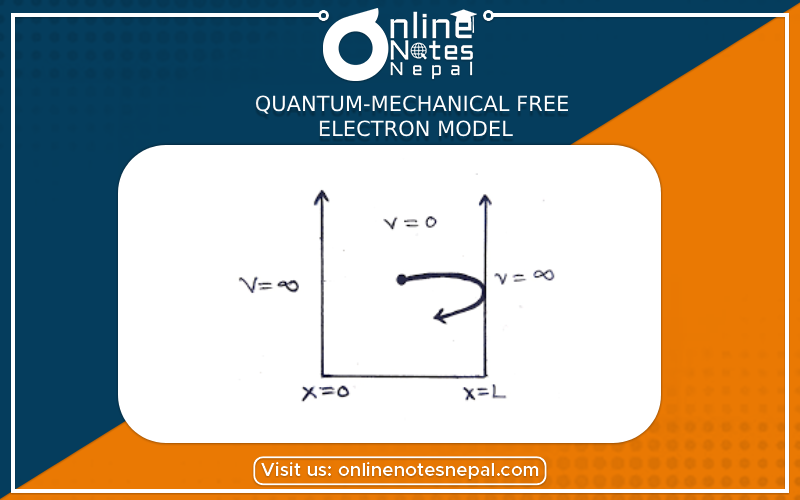
Quantum-Mechanical Free Electron Model (QMFE)
Many of the difficulties encountered by the classical free electron model were removed with the advent of quantum mechanics. In 1928, A. Sommerfeld modified the free electron model in two important ways:
As a result of these modifications, when we put an electron gas in a solid, we begin by putting the electrons in the lowest energy states available, while obeying the exclusion principle, until we have used all the available electrons. This is to be contrasted with the classical free electron gas in which Arnold Sommerfeld (1868-1951). the electrons can assume continuous energy values, with many electrons having the same energy. This has profound implications for the statistical distribution of energies that the electrons can have. Thus, whereas a classical gas will obey Maxwell-Boltzmann statistics (Supplement 9-1, Chapter 9), the quantum mechanical gas will follow a new type of statistical distribution known as the Fermi-Dirac distribution (Supplement 23-2 at the end of this chapter). This in turn will affect the way the electron gas can absorb energy from an external source, such as a heat source, and the way it responds to an electric field. Aside from these two key modifications, Sommerfeld kept most of the assumptions of the Drude model: