Published by: BhumiRaj Timalsina
Published date: 26 Jun 2021
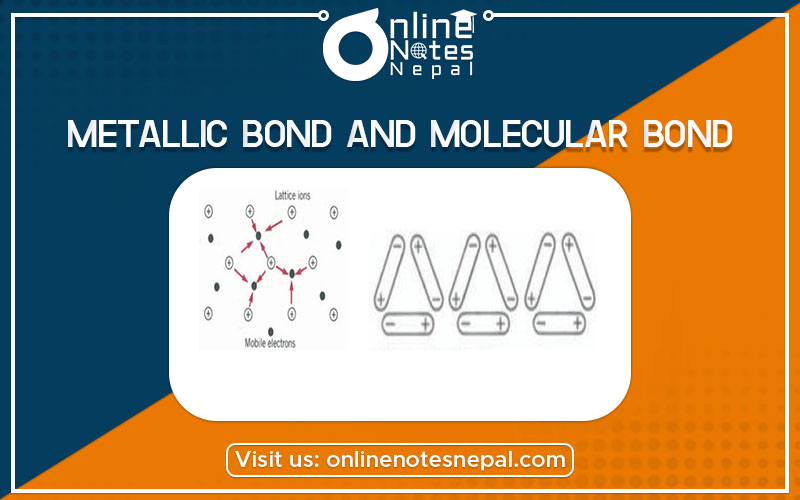
The actual crystal bonding is usually a mixture of two or three. There are four general types of bonding ionic bond, covalent bond, metallic bond, and molecular bond. The metallic bond can be thought of as the limiting case of the covalent bond in which electrons are shared by all the ions in the crystal.
We can visualize the metallic bonding as follows. The liberated electrons move through the entire crystal, visiting each positive ion at some time or other. We thus have a situation where an array of heavy positive ions is permeated by a “sea” of highly mobile negative electrons (see Fig. 22-11). It is the electrostatic attraction between the electron sea and the positive ions that prevents the breakdown of the entire structure, which would result from the repulsion of the positive ions. The electrons provide the “glue” that keeps the structure together.
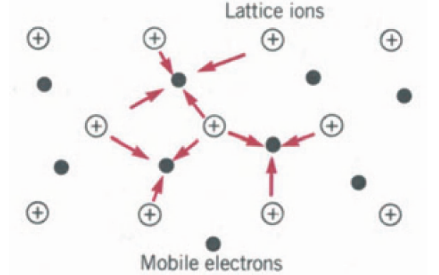
The metallic bond is in many ways similar to the ionic bond in the sense that the main role is played by the electrostatic attraction between unlike charges; however, there is a big difference. Whereas in the ionic crystal the position of the positive and negative charges and therefore the directionality of the forces is fixed, in the metallic bond they are not.
The electrostatic attraction comes from all directions. This is important because it explains why a small deformation in a nearly perfect metal crystal does not cause a fracture. Whether we compress, twist, or pull a piece of metal, the cohesive forces are still there and coming from all directions. Pure metals are ductile and malleable.
We have seen that when the outer subshells of an atom are not filled, the atom tries to fill them by associating with an atom that can supply one or more electrons. The two atoms then become bonded in the process. What happens if the atom has an outer subshell that is completely filled and difficult to excite, such as is the case with the rare gases? The answer is that it is very difficult for them to bond and therefore to form molecules and solids. However, they (except He) can form solids at very low temperatures by another mechanism. The mechanism is generally known as dipole-dipole interaction, and the resulting forces are called van der Waals or London forces.
There are molecules (polar molecules) that possess permanent electric dipole moments. An example is H20, in which the molecule is formed by covalent bonding. The two electrons from the H atoms form covalent bonds with the 2p electrons from the 0 atoms. The result is that the two H atoms as well as the 0 atoms will complete their subshells, a situation that yields a stable low-energy configuration. The resulting electron configuration, however, is not symmetric. The electron concentration around the oxygen atom makes that end of the molecule more negative than the ends where the hydrogen atoms are. The partially positive hydrogen atoms repel one another, which contributes to the angle of their bonds. The net result is that the molecule has a permanent dipole moment.
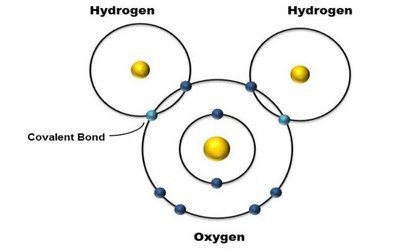
Fig -Covalent bond of the oxygen and hydrogen atoms in the water molecule (H20).
Such molecules tend to align themselves in such a way that the ends of opposite charge are adjacent and thus attract each other, forming chains and two- or three-dimensional structures
fig- Chain of molecules with a permanent electric dipole moment
A polar molecule is also able to attract molecules that do not have a permanent dipole moment. The electric field of the polar molecule causes a separation of charge in the other molecule; that is, it induces a dipole moment in the same direction as its own, as shown in Fig. The result is an attractive force.
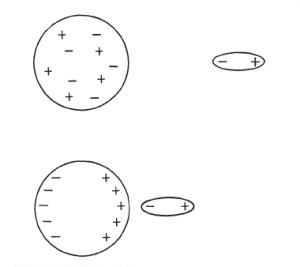
Two molecules or atoms with no permanent dipole moment, such as the rare gases, can still attract each other. Even though the electron distribution is symmetric on the average, the electrons themselves are in constant motion, and at any given instant, one part of the molecule can be more negative than another. In the polar molecule, the charge asymmetry is fixed, whereas in the nonpolar molecule there is a constantly shifting charge asymmetry. When two such nonpolar molecules are close together, their fluctuating charge distribution tends to couple and move together so that adjacent ends always have opposite signs, thus mutually inducing dipoles with a resulting attraction.
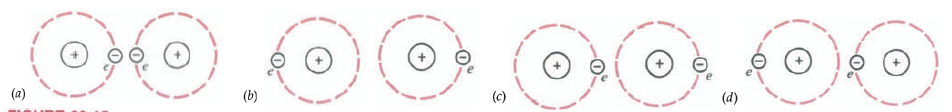
In the figure (a) and (b) Uncoupled motion of electrons in two individual atoms resulting in a situation where at times the electrons from the two atoms are on adjacent sides of the nuclei and at other times they are on opposite sides of the nuclei. (c) and (d) Coupled motion of electrons in two atoms as a result of which the electrons are never close together. This coupled motion gives rise to attractive forces between the two atoms, as can be seen from c and d. These forces are known as London or van der Waal forces.
Although London forces exist between all atoms and molecules, they can be observed in their isolated state only in the rare gases because other atoms and molecules have additional forces, discussed earlier, that mask the weaker London forces. It is these forces that cause rare gases to condense, but only at very low temperatures because the resulting binding energy of the atoms is smaller than the thermal energy at higher temperatures. All the rare gases condense, and at sufficiently low temperatures they will solidify into crystalline solids, except for helium. Helium will solidify only under high pressure and low temperature.