Published by: BhumiRaj Timalsina
Published date: 26 Jun 2021
The matter comes in three forms or phases: gases, liquids, and solids; the last two are called condensed systems. Semiconductors are crystalline solids. To understand their electrical properties we need a basic knowledge of their crystal structures.
In a molecular gas the average distance between the molecules is large compared with their size. As a result, the intermolecular forces (forces between molecules) are small compared with the forces that bind the molecules together. Because of this, the relative positions of the molecules are completely random.
The atoms and molecules in condensed systems are close together; the forces between molecules are comparable to the forces within the molecules. In a liquid the molecules form temporary short-range order arrangements that are continuously broken by the high thermal energy of the molecules. When a liquid is slowly cooled, the molecules will arrange themselves in a crystalline array that produces the maximum number of bonds and thereby leads to a state of minimum energy. If the liquid is cooled too fast, the internal energy will be removed before the molecules have a chance to arrange themselves. As a result, a solid is formed that can be considered a “snapshot” of the liquid: We have an amorphous solid that displays short-range order but no long-range order. Short-range order usually means a few atoms and long-range order thousands of atoms. Amorphous solids are called glasses or glassy solids. Because of the lack of long-range order in glasses, the bonds between different atoms or molecules vary in strength. When such a solid is heated, the weaker bonds break first, leading to a gradual softening before a complete meltdown occurs. Because of this, amorphous materials lack a well-defined melting point. We will be dealing primarily with crystalline solids and, although detailed knowledge of the crystal structure is not necessary to obtain a semi-quantitative understanding of electrical conduction processes, we will discuss briefly a few facts about crystal structures so that the structure of the semiconductor silicon can be understood.
A crystalline solid is a three-dimensional, periodic array of atoms or molecules called a crystal structure. The most convincing evidence concerning the regular arrangement of atoms in a solid comes from X-ray diffraction. The diffraction pattern not only confirms the periodic arrangement but also has allowed crystallographers to determine the arrangement. One might guess from seeing snowflakes that there is an infinite number of possible lattice arrangements, but such is not the case. It turns out that all crystal structures can be included in one of fourteen lattice arrangements.
A crystal structure can be specified by a periodic space lattice and an atom or group of atoms placed at or around each lattice point. The atom or group of atoms constitutes the basis. The space lattice is a regular periodic arrangement of points in space and is purely a mathematical abstraction. To obtain a crystal structure, we must place at or around each lattice point a basis of atoms, this group of atoms must be identical in composition, arrangement, and orientation.
One important fact about a space lattice that can be readily seen from is that every lattice point has identical surroundings. The grouping of lattice points about any given lattice point is the same as the grouping about any other point. It turns out that geometrically there are only 14 unique ways in which this can be done: This gives rise to the 14 Bravais lattices, named after the discoverer. Any lattice can be constructed by putting side by side one of these 14 Bravais lattices. These lattices are all parallelepipeds that differ from one another in the relative sizes of the three basic sides and/or the angles between them. Let us consider some crystal structures.
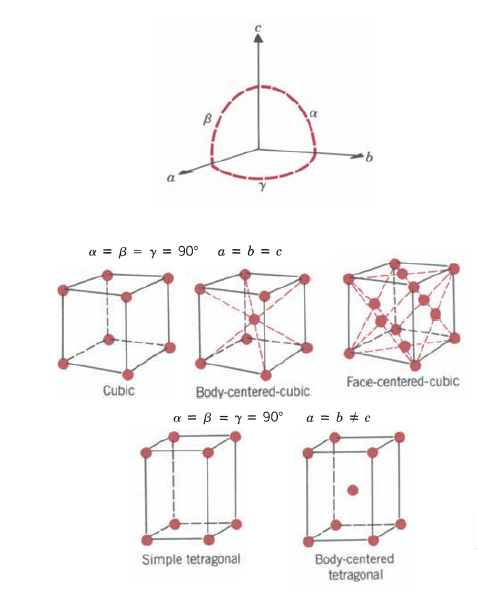
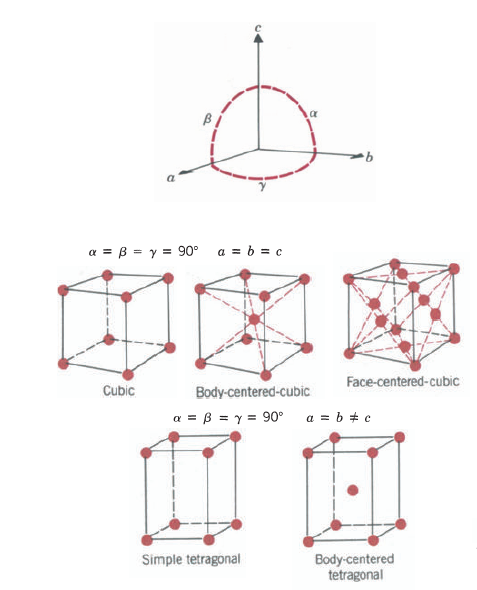
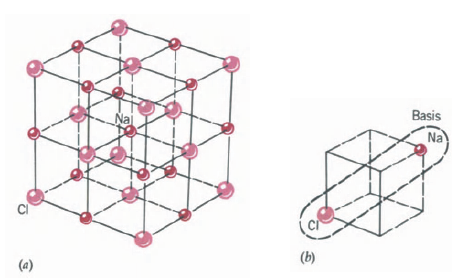
This is a face-centered cubic (fcc) Bravais lattice (Fig. 22-3a). The basis consists of a Na atom and a Cl atom separated by one-half the body diagonal, Fig, 22-3b. Other materials having the same structure include KBr, KCl, AgBr, MgO, MnO, and PbS.
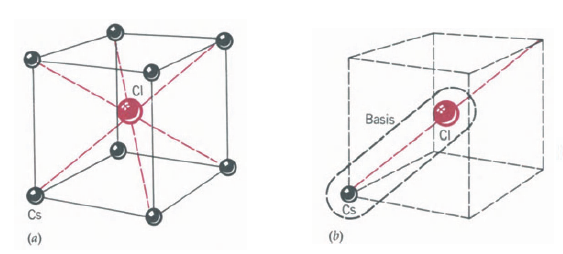
This is a simple cubic Bravais lattice (Fig. 22-4a). The basis consists of a Cl atom at the corner and a Cs atom separated by one-half the body diagonal, Fig. 22-4b. Other materials having this structure include AgMg, AINi, CuZn (brass), and BeCu.
It is a face-centered cubic. The basis consists of two identical atoms, one is situated at the corner of the cube and the other is displaced by one quarter the body diagonal along that diagonal. The diamond structure can be thought of as being made of two interlocked fcc structures, each with one atom per lattice point. These two fcc structures are displaced from each other by! d along the body diagonal.
The structure of the diamond gives rise to a tetrahedral bond arrangement where each atom can be considered to be at the center of a tetrahedron forming one bond with each of its four nearest neighbors. These appear to be located at the four corners of the tetrahedron. Materials having this type of structure include C (carbon in the diamond structure), Si (silicon), Ge(germanium), and Sn (tin).