Published by: BhumiRaj Timalsina
Published date: 22 Jun 2021
Spectrum of Hydrogen is an important piece of evidence to show the quantized electronic structure of an atom. The hydrogen atoms of the molecule dissociate as soon as an electric discharge is passed through a gaseous hydrogen molecule. It results in the emission of electromagnetic radiation initiated by the energetically excited hydrogen atoms. The hydrogen emission spectrum comprises radiation of discrete frequencies. These series of radiation are named after the scientists who discovered them.
When a hydrogen atom absorbs a photon, it causes the electron to experience a transition to a higher energy level, for example, n = 1, n = 2. When a photon is emitted through a hydrogen atom, the electron undergoes a transition from a higher energy level to a lower, for example, n = 3, n = 2. During this transition from a higher level to a lower level, there is the transmission of light occurs. The quantized energy levels of the atoms, cause the spectrum to comprise wavelengths that reflect the differences in these energy levels. For example, the line at 656 nm corresponds to the transition n = 3 n = 2.
picture
n the year 1885, on the basis of experimental observations, Balmer proposed the formula for correlating the wavenumber of the spectral lines emitted and the energy shells involved. This formula is given as:
Equation
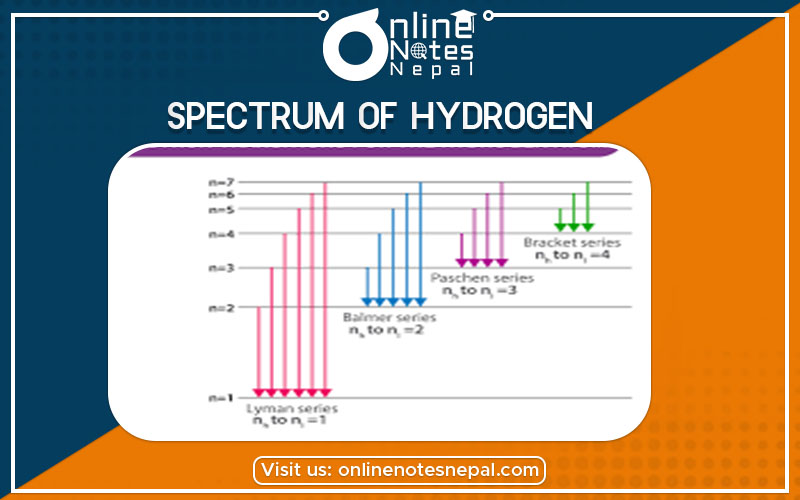
This series of the hydrogen emission spectrum is known as the Balmer series. This is the only series of lines in the electromagnetic spectrum that lies in the visible region. The value, 109,677 cm-1, is called the Rydberg constant for hydrogen. The Balmer series is basically the part of the hydrogen emission spectrum responsible for the excitation of an electron from the second shell to any other shell. Similarly, other transitions also have their own series names. Some of them are listed below,
Transition from the first shell to any other shell – Lyman series
Transition from the second shell to any other shell – Balmer series
Transition from the third shell to any other shell – Paschen series
Transition from the fourth shell to any other shell – Bracket series
Transition from the fifth shell to any other shell – Pfund series
Johannes Rydberg, a Swedish spectroscopist, derived a general formula for the calculation of wavenumber of hydrogen spectral line emissions due to the transition of an electron from one orbit to another. The general formula for the hydrogen emission spectrum is given by:
Equation
If you liked our content Boolean Algebra, then please don’t forget to check our other topics Computer System.