Published by: Nuru
Published date: 27 Jun 2021
Principle- when sparingly (slightly) soluble ionic solid is dissolved in water, it undergoes ionization, and ions forming from the solid phase pass into the solution till the solution becomes saturated and a dynamic equilibrium is established between the ions of the saturated solution and ions of solid phase at a constant temperature.
Let us consider, sparingly soluble ionic solid such as AgCl when dissolved in water undergoes ionization, and the ions forming from the solid phase pass into the solution till the solution becomes saturated.
AgCl(s) Ag+ + Cl–
Applying the law of mass action.
Rate of ionization (r1) α [AgCl (s)]
i.e. r1 = K[AgCl (s)]
where K2 is proportionality constant and [Ag+] and [Cl–] resp. At equilibrium,
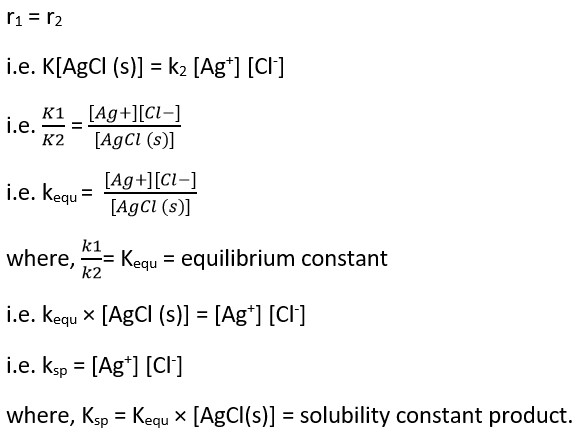
The constant is the product of concentrations of the ions of sparing solution of ionic or electrolyte or solid or salt present in its saturated solution which is constant at a constant temperature.
The value of ksp changes with the change of temperature. The value of ksp gives the idea of the precipitation of an electrolyte in the solution. A substance is out of the solution only when an ionic product exceeds it. Thus, on the basis of ionic product, the solution can be described as an unsaturated, saturated, and supersaturated solution
A substance having a low ksp value is precipitated more readily than a substance having a high ksp value. This is its principle.
Application- Its principle is very applicable in analytical chemistry. The group separation of metal in the salt analysis is done on the basis of this.