Published by: Nuru
Published date: 26 Jun 2021
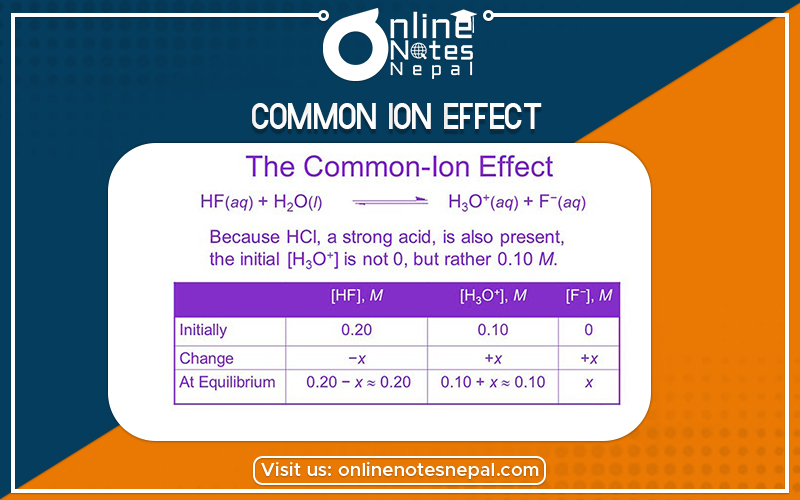
When a strong electrolyte is added to the solution of water-electrolyte [for ex- H2S, H2CO3] having ion common. The ionization of weak electrolytes is highly suppressed and this is caused by the addition of strong electrolytes to the solution of weak electrolytes is called the common ion effect.
Let us consider the ionization of NH4OH
NH4OH⇌NH4+ + OH–
Applying the law of mass action. The equilibrium constant of this reaction can be given as:-
i.e. Keq = [NH4+ + OH-] / [NH4OH]
If a strong electrolyte such as NH4OH is added to this solution, then it gives NH4+ ions as a common ion.
NH4Cl → NH4+ + Cl–
It appears to increase the concentration of NH4+ ions in the solution and the equilibrium shift towards the background direction. According to Le Chatelier’s principle to keep the value of equilibrium constants the NH4+ ion and OH– should re-combined i.e. the concentration of NH4OH should be increased.
Hence, the degree of dissociation of NH4OH is suppressed by the addition of strong electrolytes.
NH4+Cl.
Thus gives a common ion NH4+. The common ion effect of NH4Cl decreases the basic characters of NH4OH.
Thus, it is defined as the phenomenon in which the degree of ionization of weak electrolytes is highly suppressed by the addition of strong electrolytes.