Published by: Nuru
Published date: 26 Jun 2021
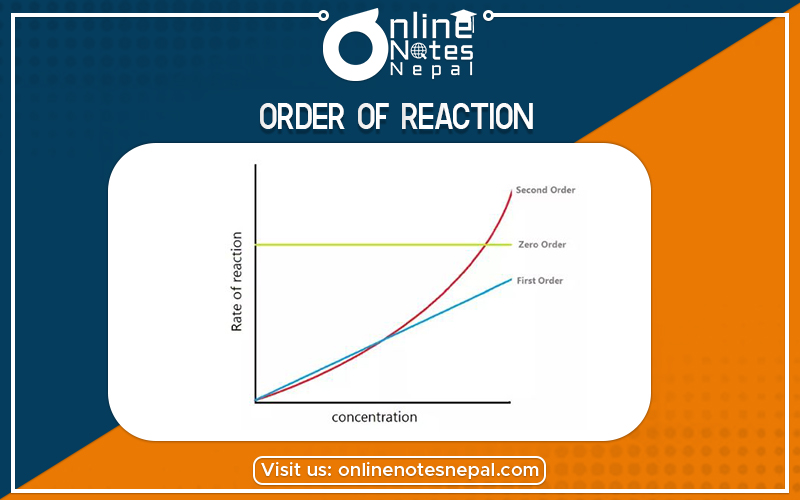
It refers to the power dependence of the rate on the concentration of each reactant. Thus, for a first-order reaction, the rate is dependent on the concentration of a single species. A second-order reaction refers to one whose rate is dependent on the square of the concentration of a single reactant (e.g., in a homo-dimerization reaction, A + A → A2) or the combined first-order dependence on the concentrations of two different reactants (A + B → C). This is distinct from the molecularity (or stoichiometry) of the reaction which is the theoretical integer value of the number of molecules involved in the reaction. For simple one-step reactions, the order and molecularity should have the same value.
The chemical reaction which appears to be of higher order but actually it is of lower order kinetics is called pseudo order reaction. It is also called pseudo unimolecular reaction. This type of reaction generally takes place when one of the reactions is in larger excess compared to another reactant.
For example,
1) Hydrolysis of the ester
RCOOR-1 + H2O ⇋ R-OH + COOH
(Alcohol) (Carboxylic acid)
Rate=K [RCOOR1][H2O]
Or, Rate = K 1[RCOOR1]
2) Inversion of cane sugar
C12H22O11 + H2O → C6H12O6 + C6H12O6
( cane sugar) (excess) (Glucose fructose)
Rate = K[C12H21O11]][ H2O]
Rate=k1 [C12H22O11]
The reaction which is interdependent on the initial concentration of reactant is called zero-order reaction.
For example
NO2 + CO + CO → NO + CO2
Rate = K[NO2]2 + [CO]0
Hence in the order of this reaction with respect to NO2 is ‘2’ and so CO is ‘0’.
Therefore overall order is 2+ 0 = 2
Some example of zero-order reactions are:
The chemical reaction in which its rate is directly proportional to the first power of the initial concentration of a single reactant is called the first-order reaction.
Let us consider the following reaction
A→ Products
Its rate law can be given as,
r= K[A]m
Where, r=rate
K= rate of constant
M= order of reaction w.r.t A
Let ‘a’ moleLit-1 be the concentration of A and x moleLit-1 of it is consumed after times ‘t’.
A→ Product
Initially ‘a’ moleLit-1
After time ’t’ (a-x) molelit-1 * molelit-1
We have
r= = K[A]m
or, = K[a-x]1
or, =K(a-x)
or, + = k(a-x)
or, = K(a-x)
or, = kdt.
Integrating on both side
-ln[a-x] =kt + c ………… (i)
Where I is the integrating constant
When x= 0 and t= 0 the equation (i) becomes
-ln(a-x) =Kt-lna
or, -ln(a-x)=kt-lna
0r, lna-ln(a-x) =kt
Or, ln ( )= kt
Or, k= ln( )
Or, k =* 2.303 log( )
Or, k= log( )………….(ii)
Equation (ii) is the required expression for integrated rate of law for 1st order reaction.