Published by: Nuru
Published date: 25 Jun 2021
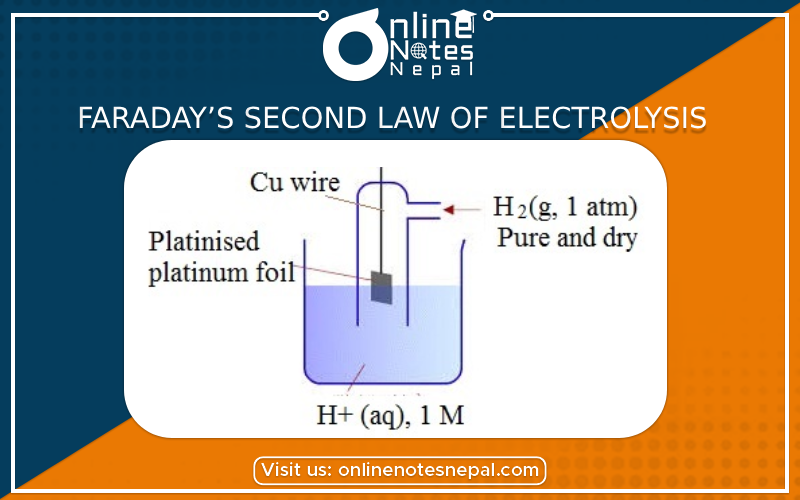
Faraday’s Second Law of Electrolysis states that when the same quantity of electricity is passed through several electrolytes.
Statement: The weight of different substances deposited at different electrodes, when the same amount of electrode, connected in series is directly proportional to their chemical equivalents or equivalent weights. The faraday’s second law of electrolysis states that, when the same quantity of electricity is passed through several electrolytes, the mass of the substances deposited is proportional to their respective chemical equivalent or equivalent weight. Mathematically, it can be expressed as:-
WαE
or, W= kE
where,
W=weight of a substance deposited
E= Equivalent weight
k= proportionality constant
pic
Explanation:-
pic
Fig- faraday’s 2nd law of electrolysis
Let us consider three breakers containing acidulated water, CUSO4 solution AgNO3 solution. These three beakers are connected in a series combination so that an equal amount of current passes through all of them. When electricity is passed through each electrolyte then hydrogen-oxygen copper and silver are deposited at their respective electrodes. It is found that when 1.008 gm of hydrogen is liberated then weights of oxygen, copper, and silver liberated are 8gm, 31.78gm, and 108gm resp. These 1.008gm, 8gm, 31.78gm, and 108gm are the equivalent weights of hydrogen, oxygen, copper, and silver resp. And hence, these data verify faraday’s 2nd law.
From this experiment:
pic
pic
Similarly,
pic
Eqn (iii), (iv) and (v) are the eqn general forms of this law of electrolysis.
Quantity of charge required to deposit one gram equivalent of the substance is called one faraday or simply faraday. The value of one faraday is nearly equal to 96,500 coulombs.
If you liked our content Faraday’s Second Law of Electrolysis, then please don't forget to check our other content Rate Law or Rate Equation