Published by: Nuru
Published date: 26 Jun 2021
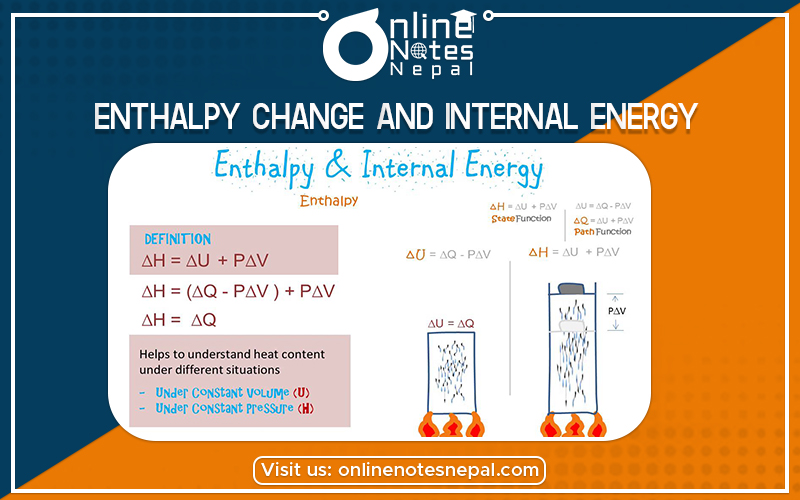
An enthalpy change is approximately equal to the difference between the energy used to break bonds in a chemical reaction and the energy gained by the formation of new chemical bonds in the reaction. It describes the energy change of a system at constant pressure. Enthalpy change is denoted by ΔH. At constant pressure, ΔH equals the internal energy of the system added to the pressure-volume work done by the system on its surroundings.
Internal energy is the sum of the potential energy of the system and the system’s kinetic energy. The change in internal energy (ΔU) of a reaction is equal to the heat gained or lost in a reaction when the reaction is run at constant pressure.
Relation Between Enthalpy Change & Change in Internal Energy
For a chemical reaction, the change in enthalpy is given as:-
ΔH= Hp – Hr………………………………..i)
Enthalpy is defined as
H= E + PV……………………………………ii)
At constant pressure
ΔH= (Ep + PVp) – (Er –P Vr)
= Ep + PVp – Er – PVr
= (Ep – Er ) + (PVp – PVr)
=(Ep – Er) + P(Vp – Vr)
=ΔE + PΔV……………………iii)
Thus, the enthalpy change of a chemical reaction is the sum of internal energy change and product of pressure and volume change. At constant volume, ΔV=0. So ΔH becomes equal to ΔE.
Let us consider the gases are ideal, we have
PV =nRT
or, PΔV = nRT
Or, ΔH –ΔE =ΔnRT (from (iii)
or, ΔH = ΔE +ΔnRT …………………..iv)
where Δn is the change in a number of moles of gas in a gaseous chemical reaction.