Published by: Nuru
Published date: 26 Jun 2021
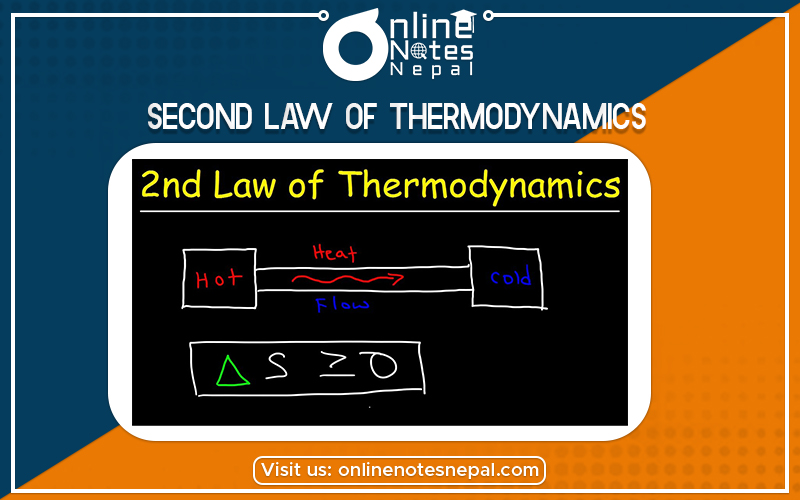
The second law of thermodynamics can be stated as: “All work can be converted into heat but all heat can’t be converted into work without leaving some changes in the system or surrounding.”
“The total entropy of the universe tends to be maximum but the total energy of the universe remains constant.”
“The entropy of the universe increases with energy spontaneous change.”
“The heat is not transferred spontaneously from a colder body to a hotter body.”
The laws of thermodynamics describe the relationships between thermal energy, or heat, and other forms of energy, and how energy affects matter. The First Law of Thermodynamics states that energy cannot be created or destroyed; the total quantity of energy in the universe stays the same. The Second Law is about the quality of energy. It states that as energy is transferred or transformed, more and more of it is wasted. The Second Law also states that there is a natural tendency of any isolated system to degenerate into a more disordered state.
It is commonly known as the Law of Increased Entropy. While quantity remains the same (First Law), the quality of matter/energy deteriorates gradually over time. How so? Usable energy is inevitably used for productivity, growth, and repair. In the process, usable energy is converted into unusable energy. Thus, usable energy is irretrievably lost in the form of unusable energy.