Published by: Nuru
Published date: 26 Jun 2021
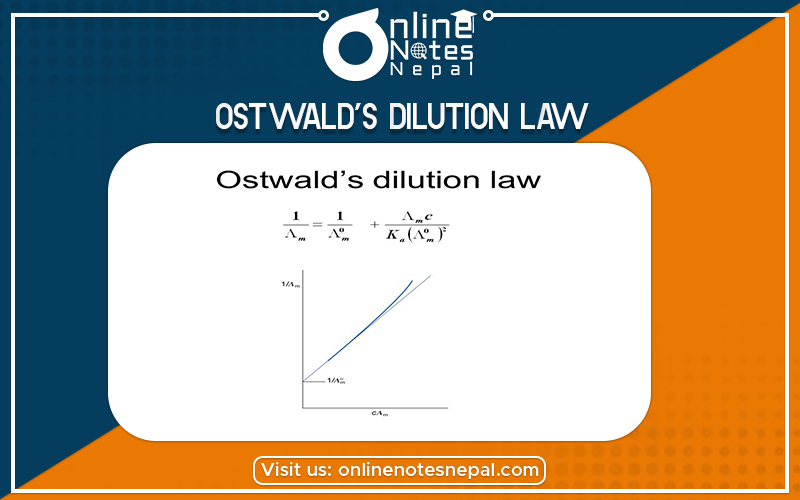
Ostwald’s Dilution Law: (Relation between degree of ionization and ionization constant)
Let us consider AB as a weak electrolyte that undergoes ionization in solution
AB A+ + B–
Let ‘α’ be the degree of ionization and ‘C’ moles per liter be the concentration of the solution. After some time 'α' of 'C' moles per liter of molecules undergo ionization.
AB A+ + B–
Initially, C mole lit-1 ֯ ֯
At equilibrium. C – Cα Cα Cα.
The equilibrium constant of this reaction can be given as
pic
This is the required expression for the relation between the degree of Ionisation and Ionisation constant.
Kequ = ionization constant
In the case of weak electrolyte, α in very small.
So, 1- α is nearly equal to 1. And now by the equation (i) becomes.
K = α2C
pic
From this equation (ii) the degree of ionization is inversely proportional to the square root of its concentration.
This equation also indicates that the degree of ionization increases to a greater extent for each dilution. So, this equation is also called Ostwald’s dilution law or equation.
The relative strength of acid and base:
In the case of weak acid lower the value of Ka (ionization constant of acid) lower will be its acidic strength, this can be explained on the basis of the following equation.
pic
In the case of weak electrolytes, the value of α is very small so, 1-α is near to ‘1’. hence we can write,
pic
Similarly, in the case of a weak base lower the value Kb lower will be its basic strength this can be explained on the basis of the following equation:-
pic
In the case of weak electrolytes, the value of α is very small so, 1-α is near to ‘1’. hence we can write as,
pic