Published by: Nuru
Published date: 26 Jun 2021
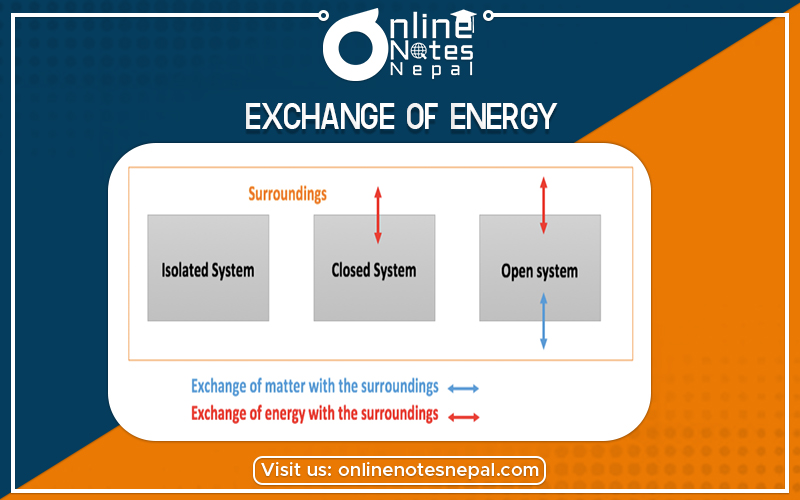
Exchange of Energy Between System and Surrounding
In thermodynamics, it is imperative to define the exchange of energy between the system and surroundings because that concept becomes the basis for many types of descriptions and calculations. A primary goal of the study of thermochemistry is to determine the quantity of heat exchanged between a system and its surroundings. The system is the part of the universe being studied, while the surroundings are the rest of the universe that interacts with the system. A system and its surroundings can be as large as the rain forests in South America or as small as the contents of a beaker in a chemistry laboratory. The type of system one is dealing with can have very important implications in chemistry because the type of system dictates certain conditions and laws of thermodynamics associated with that system.
Energy is exchanged between systems and surrounding by mainly two ways:
When systems and surroundings at different temperatures then energy is exchanged between systems and surroundings in the form of heat. If the system is at a higher temperature then that of the surrounding then energy is exchanged from system to surrounding till state equilibrium is obtained and vice versa.
If the system and surrounding area at different pressure, then energy is exchanged between the system and surroundings in the form of work.
If the gas molecules are at a higher pressure than that of the surrounding then gases push up the piston and do work against the surrounding. Here, energy is the transferred from system to surroundings.
Similarly, if the gases are at low pressure than that of surrounding then that of the surrounding then gases contract and work is done on the system by the surrounding to the system here energy is transferred from surrounding to the system,
W= -PΔV(for expansion)
W=PΔV.(for Contraction)
The direction of the flow which increases the increase of the system is given a positive sign and which decreases the energy of the system is given a negative sign.
*) work is positive when it is done on the system by the surroundings or when work is destroyed in the surrounding.
= Work is negative when it is done by the system against the surroundings or when work is destroyed in the system.
Work is not a state function because its magnitude does not depend upon the state of the system but depends upon the path by which it has been reached. The unit of work is the same as energy.