Published by: Nuru
Published date: 26 Jun 2021
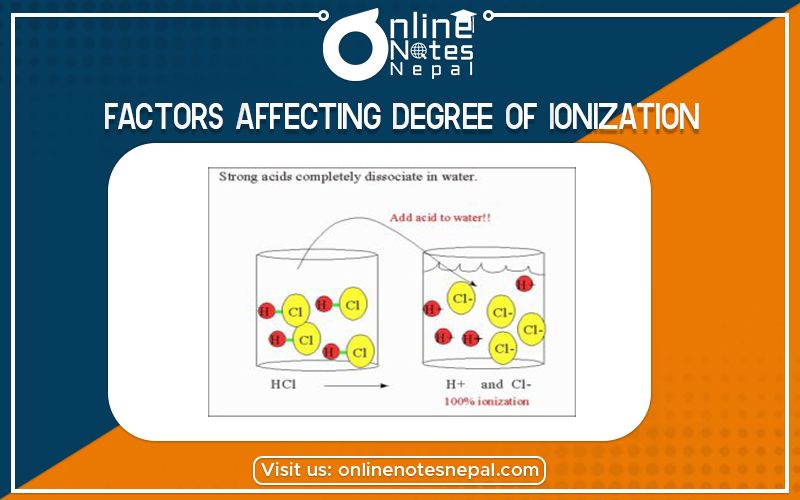
Factors Affecting Degree of Ionization is temperature, concentration, and the type of electrolytes like strong electrolyte or weak electrolyte.
Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion.
The factors of the degree of ionization are:-
The explanations of the factors affecting the degree of ionization are explained below:-