Published by: Nuru
Published date: 25 Jun 2021
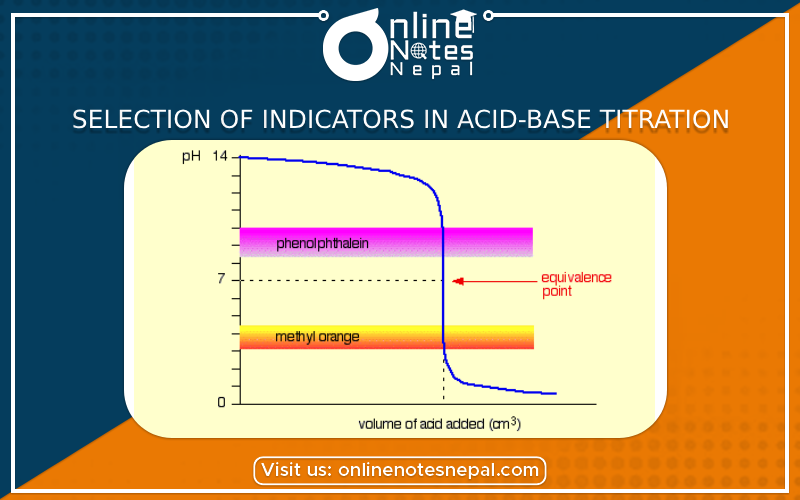
Selection of Indicators in Acid-base Titration shows the different levels of combinations of a strong base, strong acid, weak acid, and weak base.
All the indicators may not change their color in the PH range. Phenolpathlin changes its color at the PH range from 8 to 9.5 while the methyl orange changes its color at the PH range from 3 to 4.5. The PH at the equivalence point depends upon the nature of the reactions and the indicator should be selected properly areas:-
Explanations:-
During such type of titration PH value changes before and after the equivalence point. PH value changes very slowly to begin, but at the natural point. PH value changes sharply (fastly) on the addition of a small amount of base. Therefore any indicator such as either methyl orange or phemolpathaline can be used.
HCL +NaOH → NaCl + H2O
When strong acid such as HCL is titrated against a weak base such as Na2CO3, NH4OH, etc, then salt produced at the equivalent point becomes slightly acidic due to hydrolysis.
HCL +Na2CO3 →NaCl + H2O+ CO2
HCL +NH4OH → NH4Cl + H2O
For such reactions, the PH at the equivalence point becomes less than ‘7’ and for this indicator used should give the color change in the acidic medium. Thus methyl orange is only used as an indicator for such type of titration.
When the titration is carried out between weak acids such as CH3COOH and a strong base such as NaOH, then salt produced in equivalence point becomes slightly basic due to hydrolysis.
CH3COOH + NaOH = CH3COONa + H2O
Sodium acetale
CH3COONa + H2O → CH3COOH + NaOH
For such reactions, the PH at the equivalence point becomes more than ‘7’ and for this indicator used should give the color in basic medium. Thus only phenolphthalein can be used as an indicator for such titration.
When the titration is carried out between weak acids such as CH3COOH and a weak base such as NH4OH, there is no change in PH value takes place i.e. PH value remains practically constant at the endpoint of the reaction due to the formation of buffer solution.
So, no one indicator is used for such type titration.
Buffer solution
The solution which resists the PH on the addition of either acid or base is called a buffer solution. It is of two types:-
Acidic buffer solution- The buffer solution which is prepared by mixing weak acid and its salt formed by the strong base is called acidic buffer solution. for eg- CH3COOH + CH3COONa
Basic buffer solution
The buffer solution which is prepared by mixing a weak base and its salt formed by strong acid is called a Basic buffer solution. For eg- NH4OH + NH4CL
Redox titration:-
The titration involving both the oxidizing agent and reducing agent which causes both oxidation and reduction is called redox titration.
KMnO4 + H2C2O4 + H2SO4 →K2SO4 + MnSO4 + H2O + C2O (oxalic acid)
In this type of titration KMnO4 acts as an oxidizing agent and H2C2O4 oxalic acid acts as a reducing agent. This type of titration is an example of redox titration.
Acid-base titration:-
The titration involving acid and base is called acid-base titration. For eg:-
HCL + NaOH → NaCl + H2O
NH4OH + H2SO4 → (NH4)2SO4 + H2O
CH3OOH + NaOH→ CH3COONa + H2O
Therefore, the selection of indicators in acid-base titration is explained above.