Published by: Nuru
Published date: 26 Jun 2021
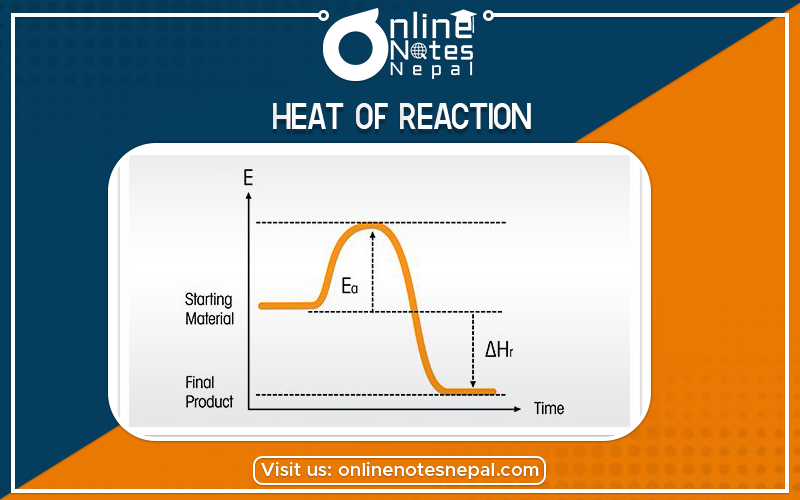
The heat of reaction at a particular temperature is defined as the amount of heat ‘q’(energy exchanged between the system and surrounding) required to return the system to the given temperature at the end. It is a thermodynamic unit of measurement useful for calculating the amount of energy per mole either released or produced in a reaction. Since enthalpy is derived from pressure, volume, and internal energy, all of which are state functions, enthalpy is also a state function.
ΔH or the change in enthalpy arose as a unit of measurement meant to calculate the change in energy of a system when it became too difficult to find the ΔU, or change in the internal energy of a system, by simultaneously measure the amount of heat and work exchanged. Given a constant pressure, the change in enthalpy can be measured as ΔH=qSee section on enthalpy for a more detailed explanation.
The notation ΔHº or ΔHºrxn then arises to explain the precise temperature and pressure of the heat of reaction ΔH. The standard enthalpy of reaction is symbolized by ΔHº or ΔHºrxn and can take on both positive and negative values. The units for ΔHº are kiloJoules per mole or kJ/mol.
ΔH and ΔHº Reaction