Published by: Nuru
Published date: 26 Jun 2021
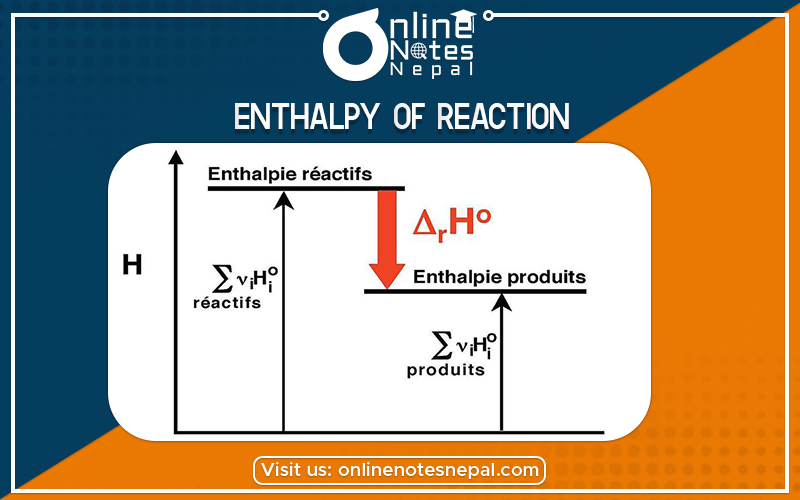
The enthalpy of reaction (ΔHRXN) is the difference between the total enthalpy of the products of a reaction and the total enthalpy of the reactants.
It is defined by the following expression.
H=E+pV
Where,
H=Enthalpy
E=Initial energy
P= Pressure
V=Volume
Thus it is defined as the sum of initial energy and product of pressure, volume, since, E, P, and V are state functions do enthalpy is also a state function. For a chemical reaction the change can be given as:
ΔH= Enthalpy of product –Enthalpy of reactant
I.e. VH= Hp –HR.
Thus in a chemical reaction of product is greater than that of the reactant then, ΔH is found to be positive which indicates heat is absorbed by the system and such a chemical reaction is called Endothermic reaction.
Similarly, if in a chemical reaction Enthalpy of product is less than that of the reactant then ΔH is found to be negative which indicated heat is evolved during the chemical reaction and such chemical reaction is called Exothermic reaction.
ΔH At Constant Pressure
From the first law of thermodynamics
q= ΔE+ P ΔV
If the chemical reaction is bought under constant pressure then, the heat of the reaction is denoted by ‘qp’.
‘qp’=(Ep – Er) + P(Vp – Vr)
= Ep – Er + PVp – PVr
= Ep + PVp – Er – PVr
=(EP + PVp) – (Er + PVr)
=Hp – Hr
qp =ΔH.
Thus, the above expression shows the change in Enthalpy of reaction (ΔH) becomes equal to the amount of heat of reaction at constant pressure (qp) and temperature.
I.e. heat evolved or absorbed means the amount of heat exchanged with the surrounding.