Published by: Nuru
Published date: 06 Jul 2021
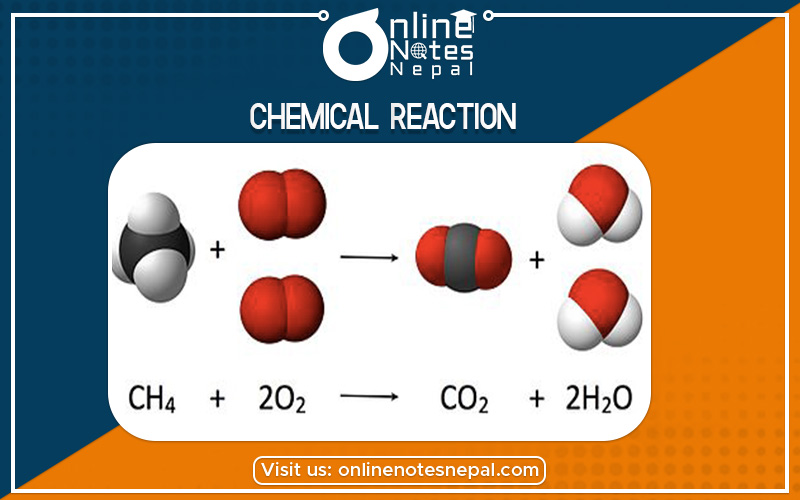
A chemical reaction happens when one or more chemicals are changed into one or more other chemicals. Examples: iron and oxygen combining to make rust. vinegar and baking soda combining to make sodium acetate, carbon dioxide and water. Many reactions that happen inside living things.
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and formulae, wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side. The first chemical equation was diagrammed by Jean Beguin in 1615.
Nitrogen + Hydrogen Ammonia
N2 + H2- NH3 ↑
Here, N2 and H2 are reactants and NH3 is the product.
If the total number of atoms in the reactants and products are the same then the chemical reaction is said to be a balanced chemical equation.
For example:
4Al + 3O2 → 2Al2O3
H2 + O2 → H2O
Here, the number of oxygen in the reactant is not equal to the oxygen in the product side. So, to balance the equation we have to balance the numbers on the reactant as well as product side.
2H2 + 02 → 2H20
Here, all the elements in the product side are equal to the elements in the reactant side.
a. Simple Contact:
When two elements are brought in contact they react. This is the simple condition under which chemical reaction takes place.
2Na + Cl2 → 2NaCl
b. Light:
Light simulates the reactants for a chemical reaction. When hydrogen reacts violently with chlorine in the presence of diffused sunlight hydrogen chloride is produced.
H2 + Cl2 →Sunlight 2HCl
c. Temperature:
Increase in temperature increases the rate of almost all chemical reactions while a decrease in temperature decreases the reaction rate. The increase in temperature increases the kinetic energy of molecules. It causes the breaking of bonds n the reactant molecules and reaction takes place at a high speed.
d. Concentration:
The rate of reaction increases with the increase in the concentration of reactants. This means that the rate of reaction is directly proportional to the concentration of the reactants. For e.g. when dilute HCl is poured into a piece of calcium carbonate there is effervescence with the evolution of CO2. The effervescence is brisk when concentrated HCl is added. So it increases the rate of the chemical reaction between CaCO3 and HCl.
e. Pressure:
Pressure is another important factor influencing the chemical reaction. With the application of high-pressure nitrogen and hydrogen combined to form ammonia.
N2 + H2- NH3
f. Catalyst:
A catalyst is defined as a chemical substance that changes the rate of chemical reaction itself without undergoing any permanent chemical change during the course of the chemical reaction. They are of two types; they are a positive catalyst and a negative catalyst.
Manganese dioxide acts as a positive catalyst in the decomposition of hydrogen peroxide without heating. This is because it increases the decomposition rate of hydrogen peroxide. Also, glycerin acts as a negative catalyst as it decreases the rate of reaction during the decomposition of hydrogen peroxide into water and oxygen.
2H2O2 →MnO2 2H2O + O2
| Types of Chemical Reactions | Explanation | General Reaction |
| Combination reaction | Two or more compounds combine to form one compound. | A + B → AB |
| Decomposition reaction | The opposite of a combination reaction – a complex molecule breaks down to make simpler ones. | AB → A + B |
| Precipitation reaction | Two solutions of soluble salts are mixed resulting in an insoluble solid (precipitate) forming. | A + Soluble salt B → Precipitate + soluble salt C |
| Neutralization reaction | An acid and a base react with each other. Generally, the product of this reaction is a salt and water. | Acid + Base → Salt + Water |
| Combustion reaction | Oxygen combines with a compound to form carbon dioxide and water. These reactions are exothermic, meaning they give off heat. | A + O2 → H2O + CO2 |
| Displacement reaction | One element takes place with another element in the compound. | A + BC → AC + B |
The molecular weight is the mass of one mole of a substance. Usually, the units used for this are grams per mole. In this movie, we show how to calculate the molecular weight of a substance from the atomic weights given on the periodic table.
The molecular weight of a substance (elements or compounds) is expressed in term of the gram, which is called its gram molecular weight. It is also called gram mole or only mole.
One mole of a substance is defined as a collection of 6.023*1023 particles of that substance.
6.023*1023 = 1 Avogadro’s number