Published by: Nuru
Published date: 06 Jul 2021
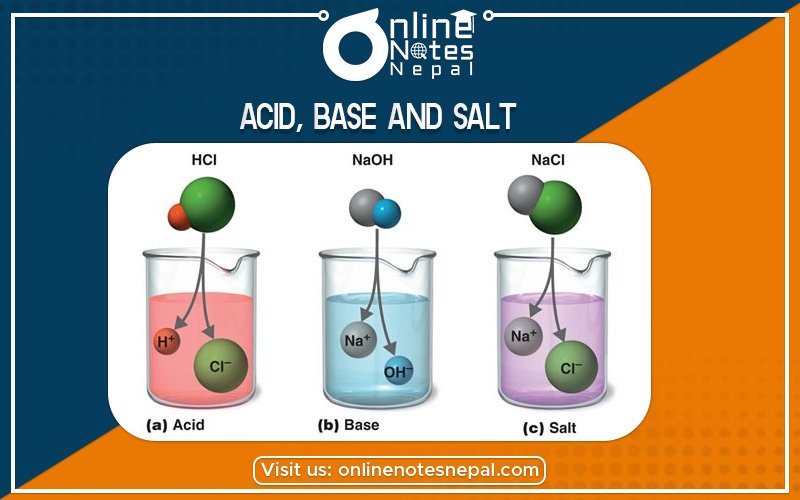
Acid: An acid is a chemical species that donates protons or hydrogen ions and/or accepts electrons. ... The word acid comes from the Latin words acidus or acere, which mean "sour," since one of the characteristics of acids in water is a sour taste e.g., vinegar or lemon juice.
Examples: - Hydrochloric acid (HCl), sulphuric acid (H2 SO4), nitric acid (HNO3), carbonic acid ( H2CO3 ) HCl H+ + Cl-
In chemistry, a base is a chemical species that donates electrons, accepts protons, or releases hydroxide (OH-) ions in an aqueous solution. Types of bases include Arrhenius base, Bronsted-Lowry base, and Lewis base. Examples of bases include alkali metal hydroxides, alkaline earth metal hydroxides, and soap.
Salt, in chemistry, the substance produced by the reaction of an acid with a base. A salt consists of the positive ion (cation) of a base and the negative ion (anion) of an acid. The term salt is also used to refer specifically to common table salt, or sodium chloride. They are neutral compounds, which are formed by the reaction between acid and base. Example: NaCl, CaCL2 etc.
Normal salt: The salt formed by the complete displacement of hydrogen atoms by a metal or electropositive radicals. They are neutral in nature. For e.g.: NaCl, KNO3, etc
Strong salt: The salt formed by strong acids and a weak base. E.g. CuSO4
Basic salt: The salt formed by weak acid and strong base. E.g.Ca (HCO3)2
They are as follows:
NaOH + HCl → NaCl + H2O
Reaction of acid on metallic oxides
FeO + 2HCl → FeCl2 + H2O
Action of acids on metal
Zn + H2SO4 → ZnSO4 + H2
The characteristics of salt are:
In the salt Na2SO4, sodium radical (Na+) is obtained from the base (NaOH) and SO4--radical is obtained from the acid H2SO4. So Na+ and SO4—are called basic radical and acid radical respectively.
Indicators are the chemical substance used to identify acidic or basic nature of the substance.
| Indicator | Acids | Bases |
|---|---|---|
| 1. Red litmus | remains red | turns blue |
| 2. Blue litmus | turns red | remains blue |
| 3. Phenolphthalein | colourless | pink |
| 4. Methyl orange | red | yellow |
A universal indicator is a special kind of indicator which is used to measure the strength of acidity or alkalinity. It is prepared by several organic indicators of different colours. It is found in green-blue solution or in the form of yellow litmus paper.
pH Scale is the scale which shows the number of hydrogen ions in acid or base.
If the concentration of hydrogen ion is less than 7, then it shows acidity of the substance and if the concentration is more than 7 then it shows the basicity of the substance.
When we add alkali on acid, the pH value of the acid goes from its acidic value to value 7 where it acts as a neutral compound as the pH value of neutral compound is 7.