Published by: BhumiRaj Timalsina
Published date: 25 Jan 2022
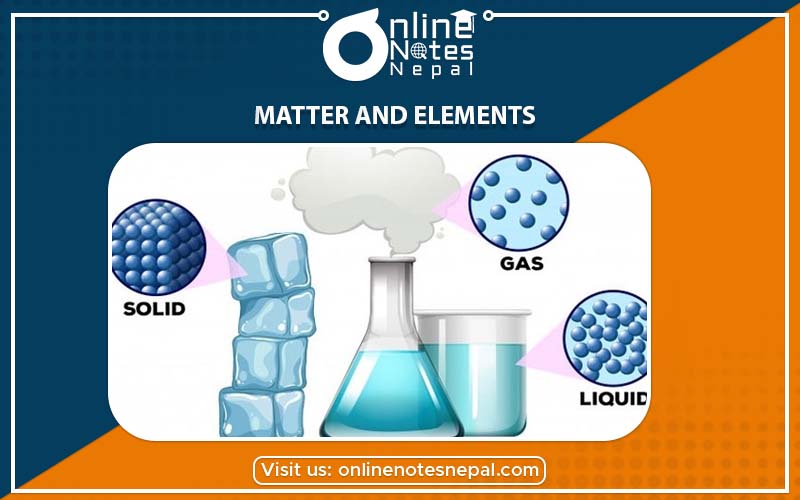
There are various types of substances in our environment like air, water, soil, stone, animals, plants etc. They have mass and volume. They occupy certain space. They are known as matter. The common definition of matter is anything that has mass and volume or occupies space. Everything around us is made up of matter. But we do not call sound, light etc matter because they do not have mass and volume.
Matter exists in three states. They are solid, liquid and gas. Some may have fixed shape whereas some may not have fixed shape. Some may conduct heat and electricity whereas some may do not conduct heat and electricity. Matter can be changed from one state to another.
Matter exist in three physical states. They are solid, liquid and gas. Bricks, stone etc are the examples of solids. Water, petrol etc are the examples of liquid and nitrogen, hydrogen etc found in the atmosphere are the examples of gas.

In the solid state, the atoms are tightly packed. They have fixed shape and a fixed volume. Some of the examples of solids are brick, stone. Some of the characteristics of solids are discussed below,

Liquid has no fixed shape but has a fixed volume. The molecules are not tightly packed as in solid. Some of the examples of liquids are water, ink, milk, cooking oil, petrol etc. Some of the characteristics of liquids are discussed below,

Air, oxygen, carbon dioxide, nitrogen, hydrogen, carbon, neon, argon etc are some common examples of gas. They do not have fixed geometrical shapes. Some of the characteristics of gas are discussed below,
Various types of elements are used in our daily life activities. Like, gold is used as jewellery, iron is used for making utensils and cooking materials, aluminum is used for making doors and windows, copper is also used for making utensils etc. Elements are pure substances. They are made of only one kind of atoms. Elements are the simplest substances. Atoms of the different element are different. The atoms of copper are different than the atoms of iron. As we know that, the elements are made of only one kind of atoms, it cannot be split into two or more simpler substances. So, an element can be defined as the pure substance made of only one kind of atoms which cannot be split up into two or more simpler substances by ordinary chemical methods.
Some of the examples of atoms are hydrogen, helium, oxygen, nitrogen, sodium, magnesium, gold, copper, iron, silver, carbon, etc. Till now, a total of 118 elements has been discovered. Out of them, 92 elements occur naturally and remaining 26 elements have been prepared artificially by scientists. Elements also exist in three states at room temperature. Elements like iron, copper, gold etc are found in the solid state. Elements like bromine, mercury, caesium etc are found in liquid state and elements like oxygen, hydrogen, etc are found in the gaseous state.
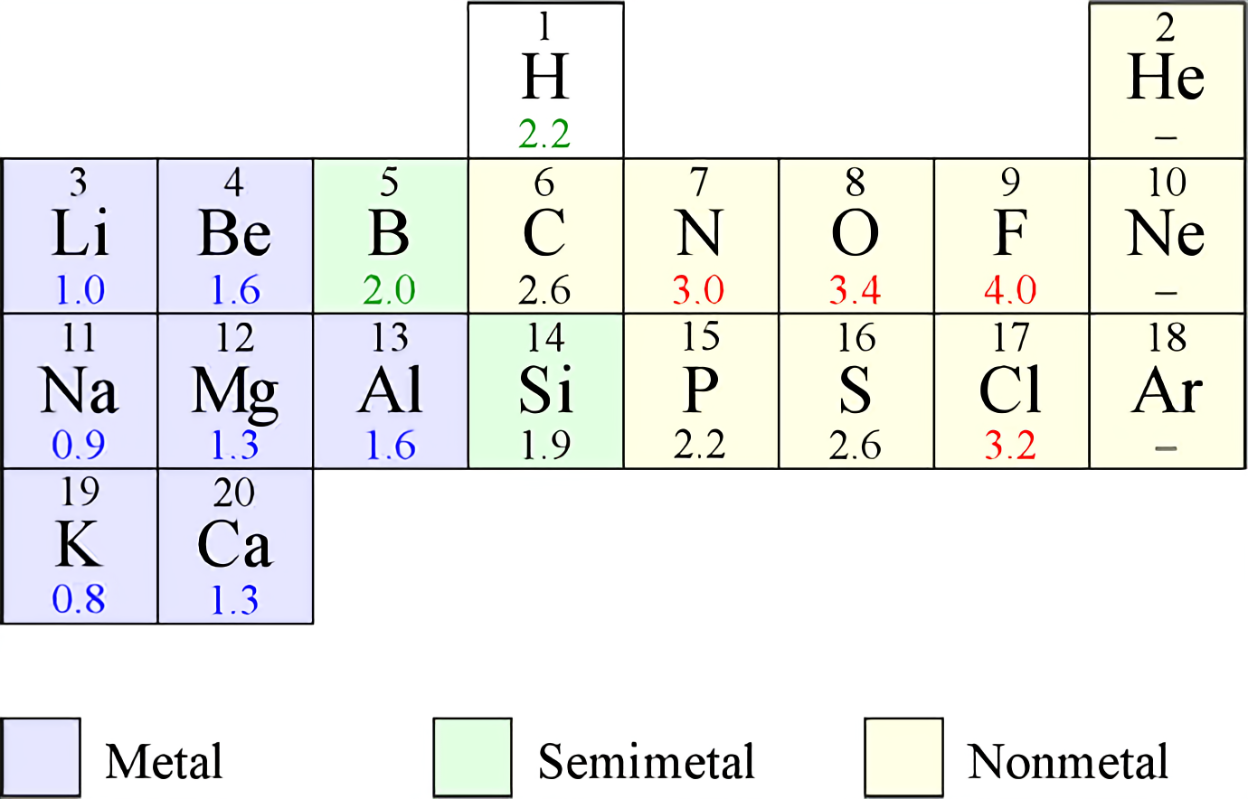
Chemical symbols are shorthand abbreviations of the names of the elements. Each element has its own unique symbol. The symbols are given to the elements according to the international agreement. They are used to write chemical reactions quickly and conveniently. Symbols are usually composed of one or two letters of the respective elements. Most of the symbols of elements are derived from their English name but only a few of them are derived from other languages.
About writing symbols, they are usually composed of two letters of the elements. Mostly, the first letter of the element is used as a symbol. Sometimes, when the two or more than two elements have a same first letter, then, in that case, one of the element is given a one-letter symbol but all other elements are given two letter symbol. The first letter is always written in capital and the second letter is written in a small letter if there is the second letter. For example, the symbol of Boron is B and the symbol of Carbon is C. Here, the symbols are written using a capital letter. The symbol of Hydrogen is H and Helium is He. Since both of them have a same first letter, Hydrogen is given symbol using first letter only 'H' and Helium is given symbol using first letter and a second letter 'He'. Here also the first letter is written in a capital letter while the second letter is written using lowercase. Some of the symbols of elements are written from their latin names. Like, the symbols of sodium. i.e. 'Na' has been derived from its Latin name Natrium and the symbols of potassium, i.e. 'K' has been derived from its Latin name Kalium.
| Atomic number | Names of elements | Symbols |
| 1. | Hydrogen | H |
| 2. | Helium | He |
| 3. | Lithium | Li |
| 4. | Berylium | Be |
| 5. | Boron | B |
| 6. | Carbon | C |
| 7. | Nitrogen | N |
| 8. | Oxygen | O |
| 9. | Fluorine | F |
| 10. | Neon | Ne |
| 11. | Sodium | Na |
| 12. | Magnesium | Mg |
| 13. | Aluminium | Al |
| 14. | Silicon | Si |
| 15. | Phosphorus | P |
| 16. | Sulphur | S |
| 17. | Chlorine | Cl |
| 18. | Argon | Ar |
| 19. | Potassium | K |
| 20. | Calcium | Ca |