Published by: BhumiRaj Timalsina
Published date: 24 Jan 2022
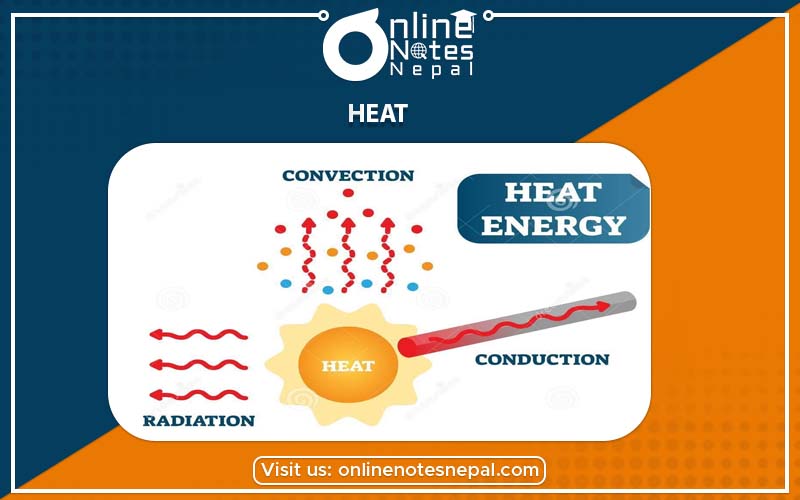
Heat is a form of energy that gives the sensation of warmth. It is obtained from hot bodies. It always flows from hot object to cold one. Heat is measured in joule, calorie etc. Coal, diesel, gas, etc. burn and produce heat energy. Heat energy is produced due to the movement of molecules. We feel hot when the sun shines. This shows that the sun is the source of heat energy and produces heat. Heat is produced from friction, electricity, chemical reactions, collisions, etc. The sun is the main source of heat energy. We use heat energy in our daily activities like for cooking, drying clothes, boiling water, producing steam to generate electricity, producing warmth, for steam distillation etc. When heat is applied to a body, it increases the volume, increases temperature and changes the state.
Heat transfer is the exchange of thermal energy between two physical systems. Heat flows from one place to another. It flows from higher temperature to lower temperature. There is the transmission of heat in our daily life activities. Heat is transferred from the sun to the earth as heat flows from higher temperature to lower temperature. Heat is transferred through various ways. Heat has several interesting ways of travelling. It travels by three ways which are given below,
Conduction is the form of heat transfer from one particle to another without the actual movement of particles that takes place within solid when one portion of the object is heated. Energy is transferred from heated area to the unheated area on the basis of the difference in temperature. Conduction takes place in solids.
For example: when we touch the side of burning stove we feel hot. The stove is made of metal and metal is a good conductor of heat. Here, heat is transfer from the heated area of the stove to the unheated area of the stove through conduction.
Molecules of solids are closely packed. When we heat the solid objects then the molecules absorb the heat energy. As it absorbs the heat energy it starts getting vibrating which disturbs the another molecule which is in close contact with it. It transfers heat energy to its neighbouring molecule which again starts vibrating and again transfers heat energy another molecule and so on. Like this, all the molecules of the solid vibrates and heat is transferred. This method of transmission is known as conduction.
Experiment
Objective: To demonstrate the transmission of heat in solids by conduction.
Materials required: Stand, iron rod, candle and Bunsen burner
Procedure: Bring an iron rod and fit it horizontally in the stand. Heat the one end of the rod with the help of Bunsen burner and put the other end of the rod in close contact (join the rod and the candle) with the candle.
Observation:
After few minutes, you can observe that the part of the candle where the iron rod is attached, melts.
Conclusion:
This experiment proves that the heat is transferred from the hot end to the cold end of a body by conduction.
This mode of transfer of heat is different from conduction. It involves the actual movement of molecules of a medium. Convection is the transfer of heat energy by the movement of heated liquids or gases from the higher temperature to the lower temperature. It takes place in liquids and gases. The molecules of gases and liquids are loosely packed and can move freely.

For example: When we heat water in a pot, the heat passes from the burner into the pot, heating the water at the bottom. After this, the hot water rises and cooler water moves down to replace it. As a result, all the water contents of the pot are heated at the same temperature. Here, transmission of the heat takes place through the actual movement of water molecules.
Experiment
Objective: To demonstrate the transmission of heat in liquids (convection).
Materials required: Water, stove, beakers.
Procedure: Take a beaker full of water. Then placed the beaker on a stove and heat the water. Heat the water until it boils.
Observation:
After few minutes the water (upper part) starts boiling though you apply the heat on the bottom of the container (lower part of the water).
Conclusion:
This experiment proves that heat is transferred from the body at higher temperature to the body of lower temperature through convection.
Radiation is the third mode of heat transfer. It does not require any medium to transfer. It is the transfer of heat from higher temperature to lower temperature without affecting the medium. We feel hot when the sun shines although the transmission mode of transfer of heat is neither conduction nor convection. Here, the mode of transmission of heat is radiation. The heat energy emitted by the hot body is called radiant heat. It also takes place in a vacuum as no medium is needed to transfer heat energy.
For examples: We feel hot when we sit near the fire due to radiation, we feel warm in our face due to sun’s heat, we get heat from the heater etc.
Due to the heat of the sun, the air gets heated during day time. As the hot air is lighter, it moves upward and the cold air moves downward replacing it. This movement of air is known as blowing of air or wind. This movement of air is called convection of air.
Thermo flask or vacuum flask is a device that preserves the temperature of hot or cold liquids for several hours. A British scientist named James Dewar invented thermos flask in 1890 A.D.
Structurally, there is a vacuum inside which reduces the heat lost due to conduction or convection. It is made of the double- walled glass vessel, outer case, cork support, stopper and a lid. The outer surface of the inner wall and the inner surface of the outer wall are silvered to reduce heat loss by radiation. Its mouth is closed by the cork that is made of a bad conductor of heat like wood, plastic, etc. The glass vessel is kept on cork pad and surrounded with an outer case. It helps to preserve the temperature of the liquids.
We can measure the temperature of the body whether it is hot or cold by touching. But we cannot measure the accurate temperature of the body. So, to measure the accurate temperature of the body, a special kind of temperature measuring instrument is used which is known as a thermometer. It is derived from the Latin word ‘thermo’ means heat and ‘meter’ means a measuring device. It is constructed on the principle that fluids expand on heating and contract on cooling. There are two types of thermometer, clinical thermometer, and laboratory thermometer. A clinical thermometer is used to measure the temperature of the human body and laboratory thermometer is used to measure the temperature of the different object.
A thermometer is made relatively small so that it is portable and cheap. It consists of a long glass tube with a thin-walled capillary tube with a glass bulb at its one end. The bulb is filled with mercury or alcohol and also in some portion of the capillary tube. The air above the thermometric liquid is removed. The other end of the capillary tube is sealed. A thick glass tube called stem protects the capillary tube. On the stem, markings are made. These markings are called graduations or degrees. It helps to read the temperature.

At first, the bulb of the thermometer is kept in contact with the body for a few minutes. By expansion and contraction of the thermometric liquid, the thermometer shows the temperature of the body. The liquid of the thermometer contracts and the level of liquids fall down to a constant value if the temperature of the body is below the room temperature and expands if the temperature of the body is more than room temperature. During expansion, the level of the liquid rises to a constant value. This value is read on the graduated scale or stem and the temperature of the body is determined.
Thermometric liquids are the fluid that has properties such as, large and uniform thermal expansion coefficient, good thermal conductivity and chemical stability, which makes it suitable for the use in thermometer. Mostly, alcohol and mercury are used as thermometric liquids.
Advantages and disadvantages of using alcohol and mercury as thermometric liquid are as follows,
The disadvantage of using mercury as a thermometric liquid is that it cannot be used in the very cold region to measure very low temperature as it’s freezing point is -39 degree Celsius.