Published by: BhumiRaj Timalsina
Published date: 25 Jan 2022

In our daily life activities, we use various types of things. Some of them are in the solid state, some of them are in liquid state and some of them are in gaseous state. But most of them are mixtures. Some of the common examples of mixtures that we use in our daily life are mixture are juice, milk and water, soil, biogas, coffee, tea etc. Air is the mixture of different types of gases like oxygen, nitrogen, carbon dioxide, water vapour, etc. The soil is the mixture of sand, stone, clay, salts and remains of living beings. Similarly, coffee is the mixture of coffee powder, water, and sugar. From all these examples we can understand that when two or more substances are mixed together in any proportion, the resulting mass is called mixture. The substances that are mixed in a mixture are known as components of the mixture. For example, in the air, the components of the mixture are oxygen, nitrogen, carbon dioxide etc and in tea, the components of mixtures are water, milk, sugar and tea leaves. The mixture may be in a different state like some may be in the solid state, liquid state or in the gaseous state. The mixture may contain solid and liquid or liquid and gas. There are many forms of the mixture. Some of them are given below with suitable examples,
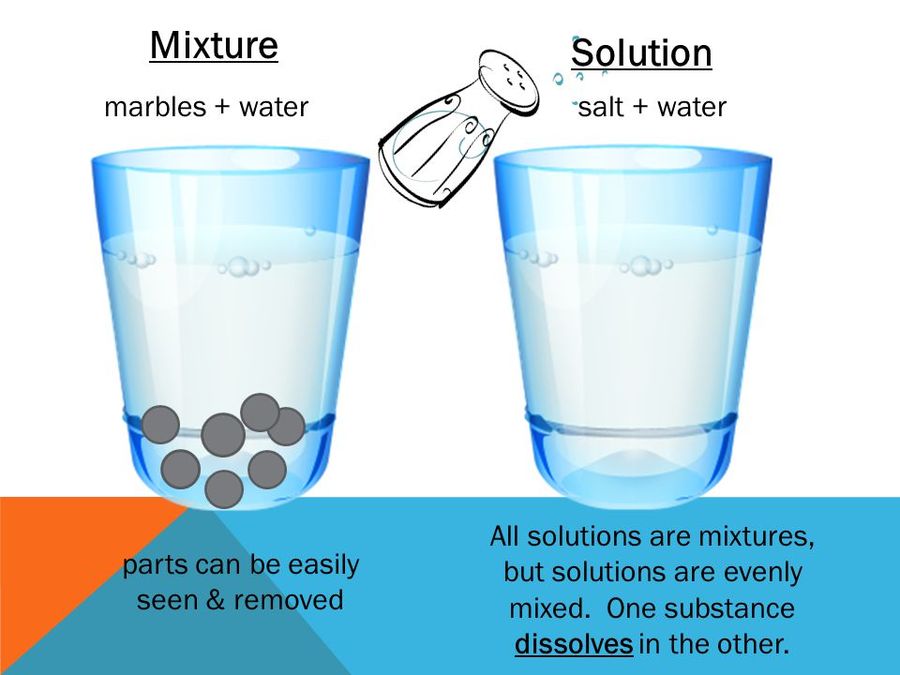
There are different types of mixtures. In some mixtures, we can see the components of the mixture through our naked eyes but in some mixtures, we cannot see the components of the mixture. They are completely dissolved. Usually, the mixture is of two types. They are a homogenous mixture and heterogeneous mixture.
Reasons for Separating the Components of a Mixture
Some mixtures may contain harmful and useless components. They may be not suitable for our daily use. Like, we remove the tea- leaves using the tea- strainer before drinking it as tea leaves are not suitable to eat. When we buy rice, pulses, etc. from the market, these things contain pieces of stone, insects and other harmful substances. We filter the tap water before drinking as it may contain harmful impurities. The main aim of separating the components of a mixture are listed below,
A solution is defined as the homogeneous mixture involving a solute and a solvent. The solute is the substance that gets dissolved and the solvent is the liquid in which the solute dissolves. Some of the examples of solvent are water, alcohol, ether, ethanol, etc and the solute is common salt, sugar, copper sulphate, etc. In sugar solution, sugar is the solute and water is the solvent. A solution of sugar and water, salt and water, copper sulphate solution etc. are the common examples of the solution.
Depending upon the amount of solute present in the solution, there are two types of solution. They are a dilute solution and concentrated solution. The dilute solution is the solution having relatively less amount of solute and concentrated solution is the solution having relatively more amount of solute. The amount of solute dissolved in a solvent determines the concentration of the solution.
There are three types of solution. They are the unsaturated, saturated and supersaturated solution. The solution that can dissolve more amount of solute at a particular temperature is called unsaturated solution. A saturated solution is a type of solution that cannot dissolve more amount of solute at a particular temperature. When we increase the temperature of the saturated solution, it becomes unsaturated and more amount of solute can be dissolved. When we cool the solution, then it removes excess solute as a solid. This solution is known as the supersaturated solution. When we increase the temperature of the saturated solution, it throws excess solute as a solid when cooled which is called supersaturated solution.
There is the various method for identifying the unsaturated, saturated and supersaturated solution. One of the methods is by using some crystals of the solute of the given solution. The solution is said to be unsaturated if the crystal of the given solute dissolves in a solution and the solution is said to be unsaturated if the crystal does not dissolve in the solution at a certain temperature. The solution is said to be supersaturated if the crystal does not dissolve and its size increases gradually.