Published by: Zaya
Published date: 25 Jun 2021
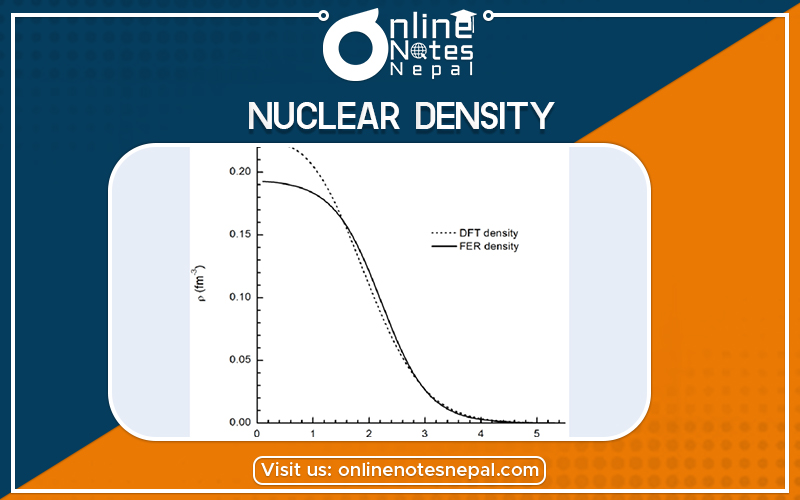
Nuclear density is the density of the nucleus of an atom, averaging about 2.3×1017 kg/m3. The descriptive term nuclear density is also applied to situations where similarly high densities occur, such as within neutron stars.
The nuclear density of a typical nucleus can be approximately calculated from the size of the nucleus, which itself can be approximated based on the number of protons and neutrons in it. The radius of a typical nucleus, in terms of several nucleons, is where is the mass number and is 1.25 FM, with typical deviations of up to 0.2 FM from this value. The density of the nucleus is thus:
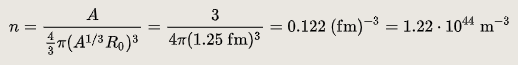
The density for any typical nucleus, in terms of mass number, is thus constant, not dependent on A or r, theoretically:
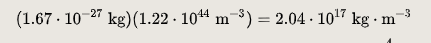
The experimentally determined value for n is 0.16 FM−3.
The mass density is the product of n by the nuclear mass. The calculated mass density, using a nucleon mass of 1.67×10−27 kg, is thus:
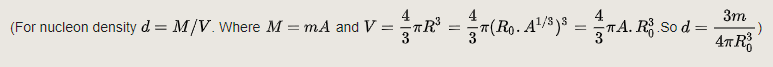

The components of an atom and a nucleus have varying densities. The proton is not a fundamental particle, being composed of quark-gluon matter. Its size is approximately 10−15 meters and its density 1018 kg/m3. The descriptive term nuclear density is also applied to situations where similarly high densities occur, such as within neutron stars.
Using deep inelastic scattering, it has been estimated that the “size” of an electron, if it is not a point particle, must be less than 10−17 meters. This would correspond to a density of roughly 1021 kg/m3.
Probing deeper within particles, one finds quarks that appear to be very dense and very hard. There are possibilities for still higher densities when it comes to quark matter, gluon matter, or neutrino matter. In the immediate future, the highest experimentally measurable densities will likely be limited to leptons and quarks.
The mass number (symbol A, from the German word Atomgewicht atomic weight), also called atomic mass number or nucleon number is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units. Because protons and neutrons both are baryons, the mass number A is identical with the baryon number B as of the nucleus as of the whole atom or ion. The mass number is different for each different isotope of a chemical element. Hence, the difference between the mass number and the atomic number Z gives the number of neutrons (N) in a given nucleus: N = A – Z.
The mass number is written either after the element name or as a superscript to the left of an element’s symbol. For example, the most common isotope of carbon is carbon-12 or 12C, which has 6 protons and 6 neutrons. The full isotope symbol would also have the atomic number (Z) as a subscript to the left of the element symbol directly below the mass number: 126C. This is technically redundant, as each element is defined by its atomic number, so it is often omitted.
The atomic number or proton number (symbol Z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The atomic number uniquely identifies a chemical element. It is identical to the charge number of the nucleus. In an uncharged atom, the atomic number is also equal to the number of electrons.
The sum of the atomic number Z and the number of neutrons N gives the mass number A of an atom. Since protons and neutrons have approximately the same mass (and the mass of the electrons is negligible for many purposes) and the mass defect of nucleon binding is always small compared to the nucleon mass, the atomic mass of an atom, when expressed in unified atomic mass units (making a quantity called the “relative isotopic mass”), is within 1% of the whole number A.
Atoms with the same atomic number but different neutron numbers, and hence different mass numbers, are known as isotopes. A little more than three-quarters of naturally occurring elements exist as a mixture of isotopes (see monoisotopic elements), and the average isotopic mass of an isotopic mixture for an element (called the relative atomic mass) in a defined environment on Earth, determines the element’s standard atomic weight. Historically, it was these atomic weights of elements (in comparison to hydrogen) that were the quantities measurable by chemists in the 19th century.|
The conventional symbol Z comes from the German word Zahl meaning number, which, before the modern synthesis of ideas from chemistry and physics, merely denoted an element’s numerical place in the periodic table, whose order is approximately, but not completely, consistent with the order of the elements by atomic weights. Only after 1915, with the suggestion and evidence that this Z number was also the nuclear charge and a physical characteristic of atoms, did the word Atomzahl (and its English equivalent atomic number) come into common use in this context.