Published by: Zaya
Published date: 25 Jun 2021
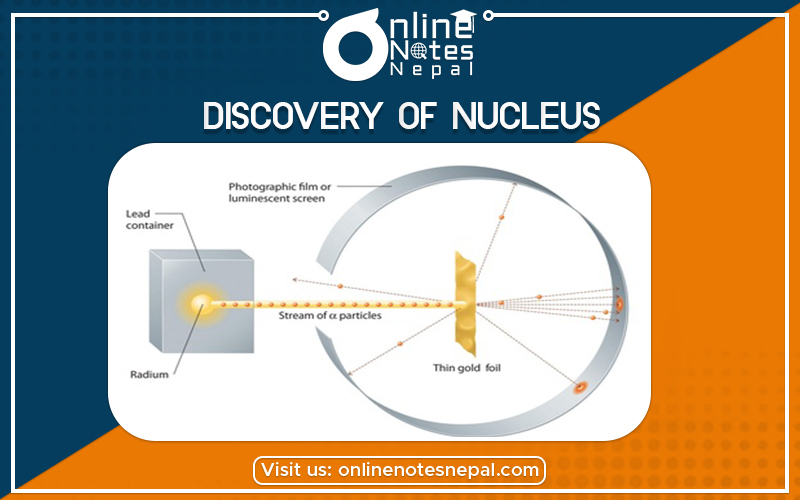
Gold sheet with the alpha particles emitted by radium. Rutherford and his students then counted the number of sparks produced by these alpha particles on a zinc sulfate screen. From this observation, they concluded that almost all the atomic matter was concentrated in a tiny volume situated at the time center, the atomic nucleus.
The discovery of the nucleus led Niels Bohr to make the first theoretical representation of the atom. The ‘quantum mechanical’ revolution, culminating in the later developments made by Erwin Schrodinger, laid the foundations for our understanding of the infinitely small.
The study of the chemical properties of the elements in the uranium-radium family showed that radium D, radium B, and radium G have the same chemical properties as lead (*). In 1913, Frederick Soddy concluding that these elements all belong in the same square of Mendeleyev’s periodic table, introduced the concept of ‘isotopes’, and won the Nobel Prize for Chemistry in 1921.
This video takes us in 1911, in Manchester, on a journey through the history of particle physics., when Ernest Rutherford conducted a historical experiment that revealed that most of the mass of an atom is concentrated in a tiny nucleus made of protons and neutrons.
This video takes us in 1911, in Manchester, on a journey through the history of particle physics., when Ernest Rutherford conducted a historical experiment that revealed that most of the mass of an atom is concentrated in a tiny nucleus made of protons and neutrons.