Published by: Nuru
Published date: 14 Jan 2022
A matter is anything that occupies space and has mass. Book, wood, pens, water etc. are the examples of matter.
The substances which is composed of its unit particles of a similar kind are known as pure substances. The substances which are composed of unit particles of different nature are known as impure substances.
An element is the simplest form of a pure substance, which cannot be split up into two or more simpler substances by any chemical reaction. All the substances are made up of elements. Hydrogen, oxygen, carbon, gold etc. are the examples of elements.
Symbol
A symbol is defined as the abbreviation of the full name of an element. Using the English alphabet symbols of elements are expressed. There are three main ways of making symbols.
1. Using the first letter of the name of elements.
| S.N | Elements | Symbol |
| 1 | Hydrogen | H |
| 2 | Fluorine | F |
| 3 | Iodine | I |
2. If the name of two or more elements begins with the same letter, another significant letter is taken with the first letter.
| S.N | Elements | Symbol |
| 1 | Helium | He |
| 2 | Lithium | Li |
| 3 | Silicon | Si |
| 4 | Zinc | Zn |
| 5 | Bromine | Br |
3. Using letters from the name of elements of their original root also make symbols. So, symbols are derived from Latin and some are from German.
| S.N | Elements | Name in other language | Symbol |
| 1 | Antimony | Stibnum (Latin) | Sb |
| 2 | Copper | Cuprum (Latin) | Cu |
| 3 | Silver | Argentum (Latin) | Ag |
| 4 | Sodium | Natrium (Latin) | Na |
| 5 | Tungsten | Wolfan (Germany) | W |
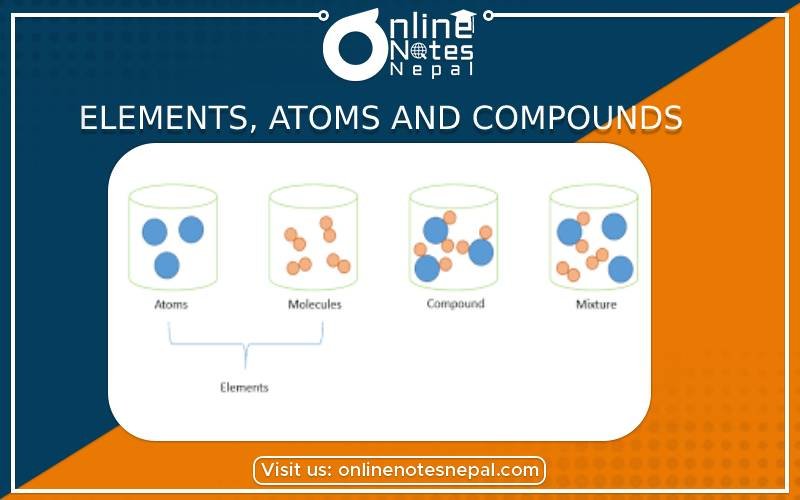
Compound is a substance of atoms of two or more elements formed by the chemical combination in a definite proportion by weight. For example, water is a liquid compound of hydrogen and oxygen in which two elements are present in a fixed ratio of 1:8 by weight. For example, water is a compound because it is made of more than one element- Hydrogen and Oxygen in the ration of 2:1. The molecular formula of water is H2O.
The smallest particle of an element or compound, which is capable of independent existence is called molecule. It retains the properties of its own. It is very small. It is difficult to estimate the size of a molecule or to determine its shape. Some of the examples of molecules are H2O (water), CO2 (Carbon dioxide), etc.
The smallest particle of an element, which takes part in chemical change is called an atom.
Atom is made up of tiny particles. These tiny particles are called sub-atomic particles or elementary particles. They are electrons, neutrons, and protons. The protons and neutrons are located at the center of an atom called nucleus. Nucleus shows the positive charge due to the presence of protons. It helps to find the atomic weight. The electrons are revolving around the nucleus in their respective orbit or shell.
Atomic number
The number of protons or the number of electrons in an electrically neutral atom of an element is called atomic number. It is denoted by Z.
Mathematically.
Atomic number (Z) = No. pf protons (p+) = No. of electrons (e-)
Atomic weight
The sum of protons and the neutrons in the nucleus of an atom is called its atomic weight. It is also called mass number. It is denoted by A.
Mathematically,
Atomic weight (A) = No. of protons (p+) + No. of neutrons (n0)