Published by: Nuru
Published date: 16 Jan 2022
The systematic distribution of an electron in different shells of an atom is called electronic configuration.
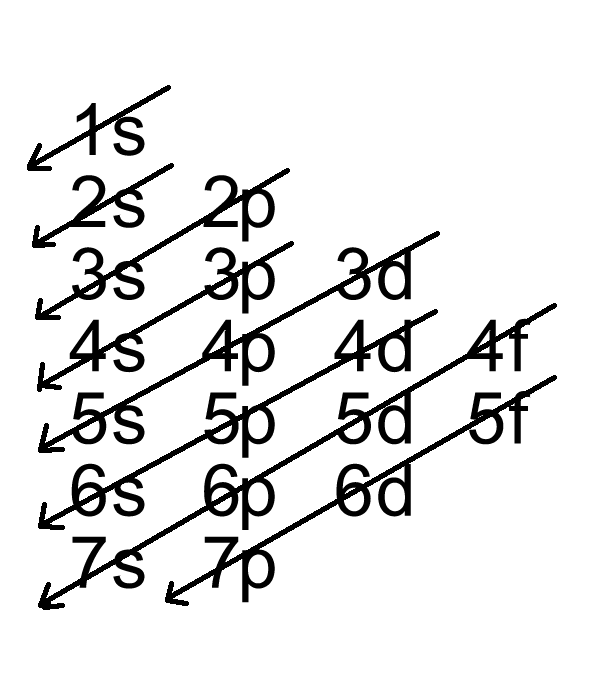
To explain the arrangement of electrons in different shells, Bohr and Bury purposed a scheme as given below:
For example:
For the K shell, the maximum number of electrons will be 2n² = 2.(1)² = 2 × 1 = 2
The energy level or shell nearer to the nucleus s called lower energy level and energy level way from the nucleus is called higher energy level.
Valence shell and Valence electrons:
The outermost orbit or shell of an atom is called valence shell and the number of electrons present in the valence shell of an atom is called valence electrons. They are far from the nucleus. The valence electrons determine the valency of an element. Valance electron takes part in the chemical reaction. The given table shows the electronic configuration and valence electron of some elements,
From valence electron, we get various information. Some of the information are given below,
Valency(combining capacity of an element):
Valency is defined as the combining capacity of element or radicle with the other element or radical to form a molecule or a compound. It is represented by numbers like1, 2, 3, 4, 5, and 6. The IUPAC has defined valency as, 'The maximum number of univalent atoms (originally hydrogen or chlorine atoms) that may combine with an atom of the element under consideration, or with a fragment, or for which an atom of this element can be substituted.'
Ways of calculating valencies of an element or a radical are listed below in points:
We use Greek prefixes to describes different valancies like mono for one, di for two, tri for three, tetra for four, penta for five and hexa for six. For example, sulphur and magnesium are divalent as they have valency two. The name of elements with the lower valency ends with a suffix- ous and that with the higher valency ends with the suffix- ic.
Some elements have changeable combining capacity. When an element shows two or more than two valencies, then it is called variable valency. For example, copper shows valency 1 in cuprous chloride (CuCl) and valency 2 in cupric chloride (CuCl2).
Following are some of the elements who show variable valency properties:
| Compound | Valency | Ion |
| Cuprous (Cu2O) | 1 | Cu+ |
| Cupric oxide (CuO) | 2 | Cu2+ |
| Ferrous oxide (FeO) | 2 | Fe2+ |
| Ferric oxide (Fe2O3) | 3 | Fe3+ |
| Element | Valency | Ion |
| Mercury (Hg) | 1 | Mercurous (Hg+) |
| 2 | Mercuric (Hg2+) | |
| Tin (Sn) | 2 | Stannous (Sn2+) |
| 4 | Stannic (Sn4+) |